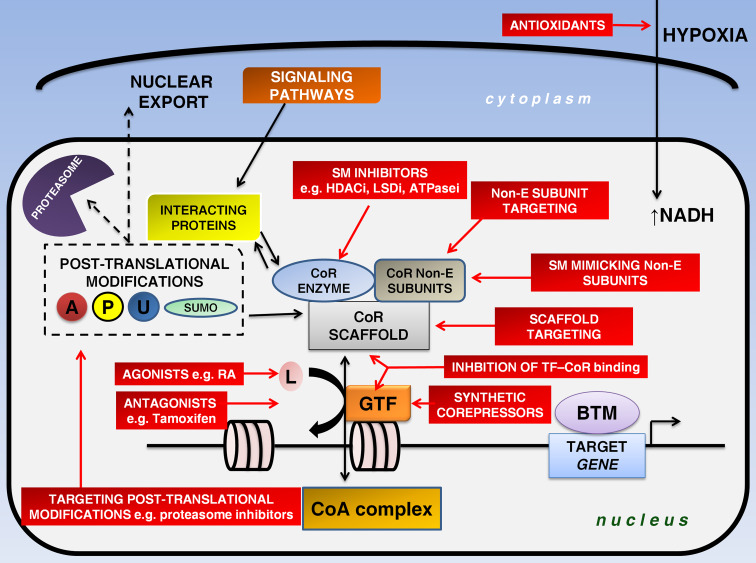
Fig. 1.
Rationale behind corepressor targeting. Corepressors fine-tune epigenetic signaling in cooperation with gene-specific transcription factors and other interacting proteins. Ligand binding regulates the competition between coregulators (repressors and activators). Post-translational modifications (acetylation, phosphorylation, ubiquitylation, sumoylation) may alter the activity of a corepressor. Red rectangles represent putative therapeutic targets. The enzymatic component of the complex is a prime target of small-molecule inhibitors. An alternative approach entails destabilization of the complex that can be achieved through targeting of the complex subunits or the use of small molecules that mimic normal subunits. Additionally, the interaction between corepressors and transcription factors may also be blocked. Recently, the generation of a synthetic corepressor was presented. Corepressor therapies seem to be more beneficial when combined with agonist or antagonist administration. A acetylation, ATPasei adenosine triphosphatase inhibitor, BTM basal transcriptional machinery, CoA coactivator, CoR corepressor, GTF gene-specific transcription factor, HDACi histone deacetylase inhibitor, L ligand, LSDi lysine-specific histone demethylase inhibitor, Non-E non-enzymatic, P phosphorylation, RA retinoic acid, SM small-molecule, SUMO sumoylation, TF transcription factor, U ubiquitylation