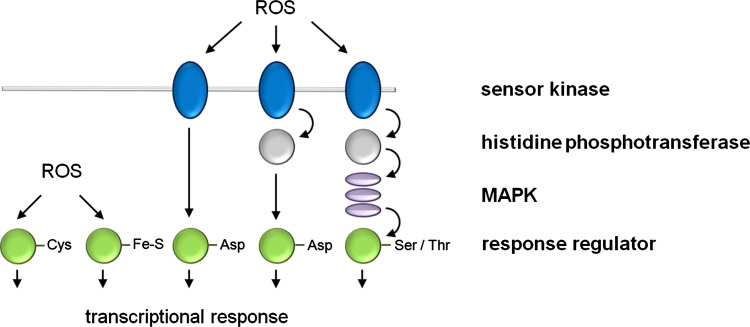
Fig. 1.
Schematic overview of the one-component and two-component signal transduction paradigm. The simplest transduction system is the one-component system (left) which is mainly found in unicellular organisms and, e.g., includes the OxyR and SoxR TFs, depicted as response regulators (RR) whose cysteine residue or Fe–S cluster, respectively, is oxidized by ROS. In the classical two-component pathway (left), the HK domain of the sensor kinase autophosphorylates and subsequently transfers a phosphoryl group to the aspartate residue of the RR. The sensory domain connected to the HK domain varies widely to allow receiving the various signals. Phosphorelay systems (second from right) are a common extension of the minimal two-component signaling cascade which in plants is involved in cytokinin signaling. Perception of a stimulus activates autophosphorylation and the phosphoryl group is intramolecularly passed to a C-terminal receiver domain. Subsequently, an additional histidine phosphotransferase (HPT) shuttles the phosphoryl group from the hybrid kinase to a soluble RR protein. Alternatively, as in plant-specific ethylene signaling or in some other higher eukaryotic organisms (rats), the phosphorelay feeds forward onto the MAPK cascade (right), which subsequently activates an RR protein via Ser/Thr residues. Taken together, one- and two-component pathways enable cells to sense and respond to stimuli by inducing changes in transcription