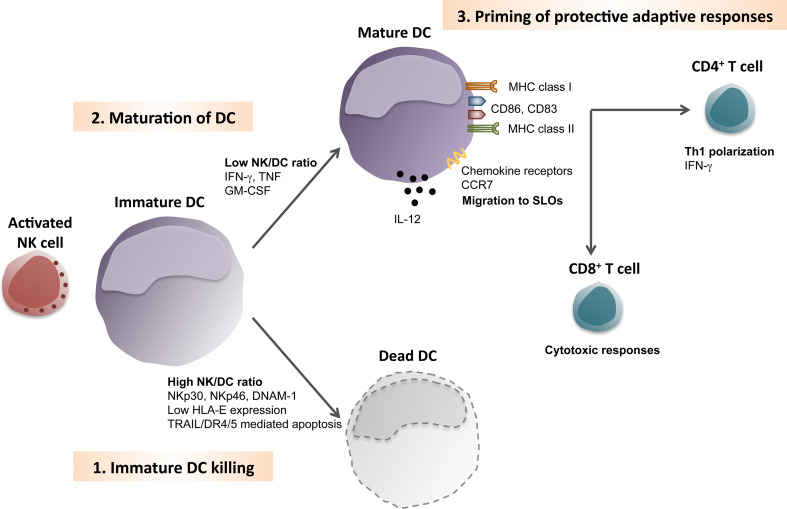
Fig. 3.
Dendritic cell editing: killing, maturation, and Th1 polarization by natural killer cells. Sites of inflammation allow activated natural killer (NK) cells to encounter immature dendritic cells (DCs). 1 At high NK cell/DC ratios, NK cells can kill immature DCs, due to the engagement of the natural cytotoxicity receptors p30 and p46 and DNAX accessory molecule 1 (NKp30, NKp46 and DNAM-1). The low levels of HLA-E expression on immature DCs are unable to inhibit this NK cell activation. It is thought that cytotoxic editing of DCs by NK cell is mediated by TRAIL/DR4/5-induced apoptosis. 2 At low NK/DC ratios, NK cells can promote maturation of DCs, via type II interferon, tumor necrosis factor and granulocyte macrophage colony-stimulating factor (IFN-γ, TNF and GM-CSF, respectively). These cytokines induce phenotypic maturation of DCs, which includes up-regulation of co-stimulatory and MHC molecules (CD83, CD86, MHC class I and MHC class II). Furthermore, in some cases, cytokine production by DCs is also induced (like IL-12 secretion). Maturation of DCs enables then to migrate into secondary lymphoid organs (SLOs), due to the upregulation of chemokine receptors, like C–C chemokine receptor 7 (CCR7). 3 Inside SLOs, DCs previously activated by NK cells were found to efficiently prime protective adaptive responses, including Th1 polarization of naïve CD4+ T cells and cytotoxic responses by CD8+ T cells