Abstract
In all complex organisms, the peripheral nerves ensure the portage of information from the periphery to central computing and back again. Axons are in part amazingly long and are accompanied by several different glial cell types. These peripheral glial cells ensure electrical conductance, most likely nuture the long axon, and establish and maintain a barrier towards extracellular body fluids. Recent work has revealed a surprisingly similar organization of peripheral nerves of vertebrates and Drosophila. Thus, the genetic dissection of glial differentiation in Drosophila may also advance our understanding of basic principles underlying the development of peripheral nerves in vertebrates.
Keywords: Peripheral glia, Schwann cell, Wrapping glia, Drosophila, Vertebrates, Septate junctions, Myelin
Introduction
Most animals rely on their ability to sense environmental signals, to compute them and to finally trigger the appropriate responses. For this task, the central and the peripheral nervous systems (CNS, PNS) with their highly sophisticated cellular adaptations have evolved. The computing is hardwired in the numerous neuronal connections and establishes an astonishing complex and interwoven lattice. As complex as the formation of such intricate networks can be, the greater challenge may lie in the fact that the neuronal ensemble has to be functional for a very long time. Neuronal signals have to be faithfully transmitted over long distances and neurons have to reproducibly elicit the required responses in their target cells over many years. Thus, the need for a tight electrical insulation and metabolic support of the nervous system is directly evident. This task is executed by a set of glial cells that ensures the comfortable life of neurons. To dissect the functional characteristics of glial cells, the PNS can be used in a simple reductionist approach, since it is mostly comprised of axons and glial cells.
The organization of peripheral nerves is relatively simple and is not complicated by synaptic connections. Thus, glial cells have to fulfill only a limited set of functional requirements. They provide trophic support to the peripheral axons [1]. In addition, glial cells insulate the different axons to allow fast electrical conductance and to participate in the establishment of the blood–brain barrier to ensure constant reaction conditions during signal propagation. In the following, we compare how glial cells differentiate in the PNS of vertebrates and invertebrates to cover these different functional needs.
Peripheral nerves in vertebrates
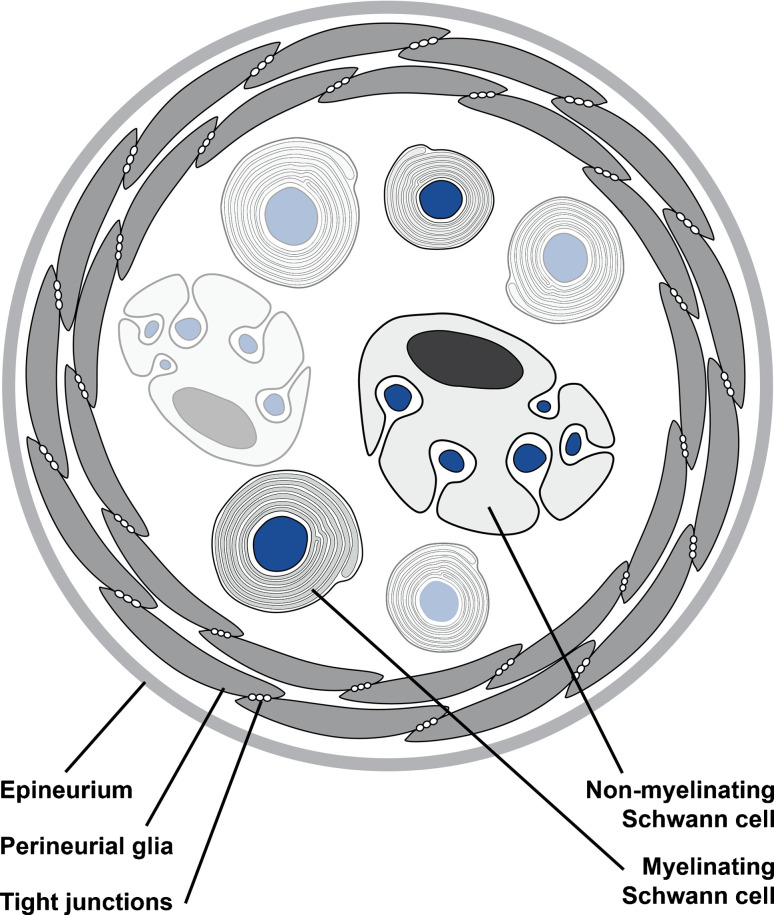
During development of the vertebrate PNS, neural crest cells detach from the dorsal neural tube and give rise to neurons and glial cells of almost the entire PNS, as well as to many endocrine cells and to other mesenchymal cells [2–4]. The glial cell complement within the peripheral nerves is comprised of myelinating and non-myelinating Schwann cells also called Remak fibers [5]. Schwann cells always ensheath a single axon whereas Remak fibers sort more than one axon into so-called Remak pockets. These cell types, together with the motor axons and the sensory axons, pericytes, endothelial and some endoneurial fibroblastic cells within the nerve, are engulfed by perineurial glial cells, which form a tight barrier between nerve and tissue fluids (Fig. 1) [6–8].
Fig. 1.
Schematic view of a cross-section through a vertebrate peripheral nerve. The tight junction forming perineurial glial cells are surrounded by an epineurium. The endoneurium hosts myelinating Schwann cells, which ensheath large caliber axons and and non-myelinating Schwann cells which engulf axons of a small caliber size
Schwann cells
Neural crest cells first differentiate into so-called Schwann cell precursors (SCPs) [9]. SCPs migrate along peripheral axon projections to reach their final position and do not seem to be important for correct axon targeting [10]. Later on, SCPs give rise to immature Schwann cells, which persist until the time of birth. Some marker genes for the different developmental stages are known (e.g., Cadherin19 as exclusive marker for SCPs; [11, 12]). Immature Schwann cells either develop into myelinating glia or non-myelinating Remak fibers. This differentiation correlates with the diameter of axons and is regulated by activation of the ErbB receptor on glial cell membranes by expression of axonal Neuregulin1 (NRG1). This not only triggers the differentiation of immature Schwann cells into either myelinating Schwann cells or Remak fibers but also determines the thickness of myelin sheath [13–15].
Perineurial cells
The mature peripheral nerves are engulfed by a layer of perineurial glial cells that will form a functional barrier providing a constant milieu for the centrally located axons and glial cells (Fig. 1). The perineurial glia forms a multi-layered sheath. This cell layer is characterized by intensive interdigitated cell–cell contacts with extensive tight junctions, which establish an efficient barrier preventing para-cellular transport of solutes. In vertebrates, the barrier function is established only 10–20 days after birth, which correlates with the detection of tight junction at the electron microscopic level [6–8, 16]. The origin of the perineurium has long been a matter of debate, but recent lineage tracing and live imaging technologies have convincingly demonstrated in zebrafish embryos that the perineurium is mostly, if not exclusively, derived from the CNS [17]. Thus, the peripheral nerves of vertebrates are comprised of neural tube and neural crest derivatives, and the interplay of the two cell types appears important for normal development.
Peripheral nerves in Drosophila
The organization of the Drosophila peripheral nerves is surprisingly similar to the one in vertebrates and can be used as a model for peripheral nerves (Fig. 2). The cellular complements of the segmentally arranged peripheral nerves are well known and all lineages have been described [18, 19]. In every abdominal segment, 30 motor neurons are born that send their axons to the lateral musculature [20, 21]. In the lateral body wall of the Drosophila larva, 42 sensory neurons are generated in each abdominal hemisegment [22, 23]. They all project their axons towards the ventral nerve cord and fasciculate with the motor axon tracts [22]. The axons are engulfed by several layers of glial cells [24]. After completion of embryogenesis, only 12 glial cells populate every segmental nerve. Seven of these glial cells originate from neuroblasts located in the ventral nerve cord and thus have to migrate along motor axons towards their final destinations. The remaining five glial cells are born in the periphery [18].
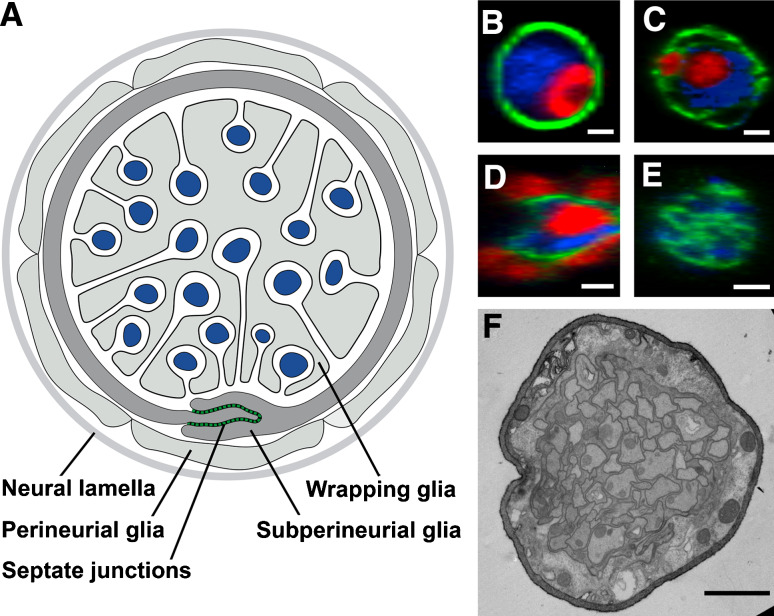
Fig. 2.
Drosophila peripheral nerve. a Schematic drawing of a cross-section of a third instar larval peripheral nerve. Perineurial glial cells are covered by an extracellular matrix called neural lamella. b–e Orthogonal cross-sections of segmental nerves. GFP-expression is shown in green, glial nuclei express Repo (red), axonal membranes are labeled by HRP staining (blue). b The neural lamella is marked by the GFP-gene trap insertion into the viking gene, which encodes Drosophila CollagenIV. c The perineurial glial cells are labeled in c527Gal4; UASCD8GFP flies. d The septate junction forming subperineurial glial cells express the moodyGal4 driver (genotype: moodyGal4; UASCD8GFP). e Wrapping glial cells are marked by expression of the nervana2Gal4 driver (genotype: nrv2Gal4; UASS65TGFP). f Electron micrograph of a cross-section through a larval peripheral nerve at the third instar stage. Scale bars 2 μm
The outermost layer of the peripheral nerve is covered by a thick extracellular matrix deposited by macrophages circulating in the hemolymph (Fig. 2) [24, 25]. Below this matrix are the perineurial cells that, unlike the perineurium of the vertebrate nerve, do not appear to form special junctional cell–cell contact structures [24]. Instead, these are found between the subperineurial cells that are highly interdigitated with pronounced septate junctions. Within the nerve are the wrapping glial cells, which towards the end of larval development have engulfed every single axon. Markers to follow the different glial cells in wild-type and mutant backgrounds are available as the result of extensive enhancer trap and exon trap experiments (Fig. 2).
Wrapping glia
The innermost glial cells of the peripheral nerve are the wrapping glial cells. During embryonic development, three to four cells are found along the nerve, but the cells have not yet initiated their differentiation [24]. Towards the end of larval development, these glial cells enwrap every single peripheral axon in a way very similar to Remak cells. Within the peripheral nerves, the wrapping glia can be specifically identified using the nervana2Gal4 driver. nervana2Gal4 was generated by using a 7-kb promoter fragment from the nervana2 (nrv2) gene, which encodes one of the 3 β-subunits of the P-type Na/K-ATPase [26]. It was originally reported as a neuronal marker but was later noted as being expressed in a large subset of glial cells [26–30]. In our hands, this Gal4 driver line and two additional GFP exon trap insertions, that faithfully mimic the endogenous nrv2 expression pattern (line173 [24] and ZCL2903 [31]), show a surprisingly strong expression in the wrapping glia of the peripheral nerves, in addition to a weak expression in the subperineurial glia that build the blood brain barrier. No expression can be detected in the glia of the eye imaginal disc.
The wrapping glia tightly associates with axonal membranes. Previously, septate junctions between axons and non-neuronal cells were described at the nerve endings, close to the neuromuscular junctions [32]. However, we have failed to detect such junctional structures along the peripheral nerves.
Subperineurial glia
The subperineurial glial cells are the main constituents of the Drosophila hemolymph brain barrier, which corresponds to the blood–brain barrier (BBB) [24, 33–35]. They form a single cell wide, squamous epithelia-like structure with highly interdigitated cell–cell contacts. At the end of embryogenesis, extensive septate junctions are generated along these interdigitated cell contact sites, which morphologically resemble the paranodal septate-like junctions. The subperineurial glial cells do not divide; however, endoreplication is likely since the nuclei of the subperineurial glial cells are very large. These cells can be labeled throughout development using the markers moodyGal4 and gliotactinGal4 [33, 36–38]. The moodyGal4 driver has been generated using 2.4-kb large promoter fragment of the moody gene and has been shown to rescue the mutant phenotype. moody encodes a G-protein-coupled receptor (GPCR) and transmits a still unknown signal regulating the formation of septate junctions (see below). In moody mutants, the length of the septate junctions is reduced, which might cause the leaky BBB phenotype. However, this may not necessarily be the direct cause of the leakage of the BBB, since mutant yurt animals also lack a functional barrier but show morphologically intact septate junctions [33, 36, 39–41].
One additional signaling pathway that has been recently identified to control the differentiation of the subperineurial glial cells in Drosophila may be defined by the PAK-like serine-threonine kinase Fray. Fray is required for the establishment or the maintenance of the axonal ensheathment by wrapping glia [42]. In fray mutants, peripheral nerves develop normally during embryonic development but exhibit severe swellings during larval stages [42]. The mammalian homolog of fray (PASK) directly phosphorylates the Na–K-2Cl co-transporter, and thereby activates solute transport, suggesting that the Fray kinase regulates ionic homeostasis [43].
Rescue experiments indicate that fray acts in the subperineurial glia and point to the important role of these cells in defining ion homeostasis in the nerve preventing severe nerve swellings. In addition, fray may control axonal ensheathment by the wrapping glia in a non-cell-autonomous manner; however, electron microscopic data are still missing [42]. The signaling cascade involved is presently unclear.
The subperineurial glial cells may also influence the development of the perineurial glial cells. Upon activation of the Ras effector phosphatidylinositol 3-kinase (PI3 K) and its downstream kinase Akt in the subperineurial glia (gliotactinGal4), the perineurial glial cells enlarge. This process depends on the FOXO transcription factor, suggesting that Ras-PI3 K-Akt signaling pathway in the subperineurial glia promotes growth of the perineurial glia [44].
Perineurial glial cells
The perineurial glial cells have the ability to divide, but currently no function is assigned to this cell type either in the PNS or in the CNS [24, 45]. Unlike the other peripheral glial cells, the perineurial glia does not contact neurons [24, 45]. Possibly, the perineurial glia exerts an accessory function during BBB formation [24] or these cells might serve as a reserve pool for structural plasticity. The best marker to label the perineurial glial cells is the Gal4 enhancer trap insertion NP6293 [45], which carries an P[Gal4] insertion within the basigin locus. Basigin encodes an Ig-domain adhesion protein that, interestingly, is involved in the neuron–glia interaction in the optic ganglia of Drosophila [46]. The perineurial glial cells abut the extracellular matrix and Basigin has been reported to interact with Integrin signaling [47].
Molecular control of axonal wrapping
In vertebrates, SCPs can either differentiate into myelinating or non-myelinating glial cells. This depends on the activity level of two members of the EGF-receptor tyrosine kinase family, ErbB3 and ErbB4. ErbB signaling results in the activation of two downstream signaling cascades: the PI3 K pathway and the Ras/MAPK pathway [48]. The initiation of myelination appears to be at least in part controlled by the PI3 K pathway and is not influenced by the Ras/MAPK pathway [49]. In mice, the activating ligand of the ErbB receptors is Neuregulin1 (NRG1). Genetic analysis demonstrated that the level of axonal NRG1 determines the extent of myelination. Reduced levels of NRG1 result in hypomyelination, whereas increased NRG1 expression leads to hypermyelination. Interestingly, the NRG1-ErbB pathway does not exclusively regulate myelination but also affects the sorting of axons into Remak fiber pockets [13]. Moreover, together with Sox2, Pax3 and Laminin, NRG1 induces cell proliferation of SCPs and can act as a survival factor for SCPs [11]. Recently, direct forward genetic screens in zebrafish are revealing further molecules underlying glial differentiation [50, 51].
In Drosophila, axonal wrapping is regulated similarly to vertebrates. Axonal wrapping has been well studied using the example of the developing compound eye, where FGF-receptor signaling regulates proliferation, migration and differentiation of glial cells [52, 53]. Most of the Drosophila compound eye is formed from the imaginal disc epithelia [54]. The only exception is seen in the retinal glial cells, which are derived from progenitor cells located in the larval CNS. These progenitor cells proliferate and migrate onto the eye imaginal disc as neuronal differentiation proceeds in this epithelium [52, 53, 55–59]. Glial cell proliferation and migration is regulated by a sustained activation of the FGF-receptor Heartless. Heartless, which is expressed specifically in the retinal glial cells, becomes activated by the FGF8-like ligand Pyramus. Interestingly, Pyramus is also expressed in the glia and thus the FGF-receptor is activated by paracrine or possibly autocrine mechanisms. Heartless activity is then transmitted to the nucleus via the function of Rap1, a small GTPase of the Ras family [52]. Once the glial cells have reached the forming photoreceptor neurons, they contact the nascent photoreceptor axons. This triggers the differentiation of glial cells into a wrapping glial cell type in a Heartless-dependent manner. However, now Heartless becomes activated via the neuronally expressed FGF8-like molecule Thisbe [52]. In conclusion, the sequential activation of a single FGF-receptor in glial cells first triggers proliferation and then stimulates differentiation.
How is this differential response to a seemingly very same signal, namely the activation of the Heartless receptor, controlled during the life of a glial cell? The first clue towards an understanding of this question stems from the observation that prior to glial differentiation, photoreceptor axons and glial cells contact each other for the first time. Concomitantly, the expression of Sprouty, a negative regulator of receptor tyrosine kinase signaling, is activated [52]. Thus, neuron–glia interaction appears to attenuate the intensity of FGF-receptor activity and sets the stage for a differential response to FGF-receptor signaling. The dampening of the FGF-receptor activity in differentiating glial cells may be of more general relevance, since we recently identified a novel negative regulator of heartless signaling expressed in glial cells in response to axonal contact (F. Sieglitz and C.K., unpublished). The combined action of these negative regulators ensures reduced levels of FGF-receptor activity and results in the inactivation of Rap1. Subsequently, target genes required for glial differentiation are then activated. Interestingly, the level of FGF-receptor activity is still decisive in setting the amount of glial wrapping of axons and more FGF-receptor activity can induce more wrapping [52].
In general, however, a typical invertebrate wrapping glial cell will only wrap around any axon once. Structures such as myelin are thought to be an invention of the vertebrate lineage. Nevertheless, myelination and even the formation of nodes have been described for annelids, malacostracan crustaceans and copepods [60–68]. In most cases, motor axons involved in escape responses are wrapped multiple times, but for some shrimps even sensory axons are additionally myelinated [65]. The tight wrapping of axonal segments induces the question how the propagation of the action potential is regulated in the invertebrate system. For some invertebrates like Aplysia voltage-gated ion channels are reported to be unevenly distributed and show clustering along the axon [69]. In cultured neurons from Manduca pupae, high expression of a voltage-gated sodium channel is noted in the axon close to the cell soma with more even expression along the remaining axonal segment [70]. In the larval nervous system of Drosophila, a similar distribution of the voltage-dependent Na+ channel Para has been observed (I.S. and C.K., unpublished).
In conclusion, although in the Drosophila nervous system myelin-like wrapping of axons is not used to facilitate faster electrical conductance, the developmental program underlying the formation of such multiple membrane wraps may be be evolutionary ancient. Receptor tyrosine kinase activity regulates the extension of glial membranes around axons in flies and mammals. Possibly, the single ensheathment is primarily needed to provide enough trophic support for the different axons [1].
The blood–brain barrier and septate junctions
In the vertebrate system, the peripheral nerves are engulfed by the perineurium, and in invertebrates, such as Drosophila, the peripheral nerves are surrounded by a layer of perineurial and subperineurial glia (Figs. 1 and 2). Not much is known on the definition of these particular lineages but it is without doubt that these cells fulfill important tasks in setting the blood–brain barrier by establishing tight junction (vertebrates) or septate junctions (invertebrates).
Formation of septate-like junctions in vertebrates
In the vertebrate peripheral nervous system, saltatory conduction requires gaps between the myelinating cells called the nodes of Ranvier. The action potential is evoked in this small area which represents 0.1–0.3% of the surface of the entire axon. The concentration of voltage-gated ion channels restricts the ion flux needed for generation of action potential to a very small area. The tight association of the myelin sheath to the axon at paranodal regions flanking the node is critical for proper saltatory conductance and is characterized by a series of septate-like junctions [71–74]. The septate-like junctions function similarly to tight junctions and restrict paracellular transport of small molecules and ions. Septate-like axo-glial junctions, however, do not prevent the diffusion of lanthanum into the periaxonal space and hence do not provide a “tight” seal as do septate junctions found in the invertebrate nervous system [75, 76]. Nevertheless, many of the molecules that are associated with septate-like junctions have been highly conserved during evolution (Table 1 [77–79]).
Table 1.
Molecular composition of different cellular junctions
| Septate junction | Septate-like junction | Tight junction | References |
|---|---|---|---|
| NeurexinIV | Caspr | [98, 132] | |
| Gliotactin | Neuroligin | [38, 133] | |
| Neuroglian | NeurofascinNF15 | [134] | |
| Megatrachea | Claudin | [135, 136] | |
| Sinuous | Claudin | [136, 137] | |
| Kune-kune | Claudin | [136, 138] | |
| Nervana2 | [100, 101] | ||
| Fasciclin II | [139] | ||
| Fasciclin III | [140] | ||
| Boudin | [141] | ||
| Coracle | 4.1 Protein | [140] | |
| Dcontactin | Contactin3 | [83, 84] | |
| Lachesin | [142] | ||
| Varicose | [143] | ||
| Yurt | [39, 40] | ||
| Disc Large | Dlg1 | [144, 145] | |
| Scribble | Scribble | [146, 147] | |
| Lethal giant larva | Hugl-1 | [148] | |
| Occludin | [149] | ||
| Tricellulin | [150] | ||
| JAMs (A-C) | [151] | ||
| JAM4 | [152] | ||
| CAR | [153] | ||
| ESAM | [154] | ||
| MarvelD3 | [155] | ||
| Polychaetoid | ZO-1, ZO-2, ZO-3 | [156, 157] | |
| MAGI-1, MAGI-3 | [158] | ||
| MUPP1 | [159] | ||
| PATJ | [160] | ||
| Cingulin | [161] | ||
| JACOP/cingulin-like protein 1 | [162] | ||
| aPKC-baz-Par6 complex | aPKC-Par3-Par6 complex | [163] | |
| ZONAB | [164] |
Axonal Caspr and Contactin form a tight cis-complex required for the expression of Caspr at the cell surface and for the localization of Contactin at the paranode [80–87]. This axonal complex binds to glial Neurofascin155 although the precise means of interaction remain unclear and may involve other proteins or protein complexes [85, 87, 88]. Animals with loss of function mutations affecting septate-like junction components fail to form the paranodal junctions [89–91]. Additionally, the nerve conduction is significantly slower and intracellular organelles accumulate suggesting that normal axonal transport depends on the integrity of the paranodal junctions [79, 92]. Interestingly, loss of several other proteins like Netrin-1 or Deleted in Colorectal Cancer (DCC), CD9, ceramide galactosyl transferase and cerebroside sulfotransferase results in a similar destabilization of the paranodal complex, which indicates a rather complicated establishment of septate-like junctions in vertebrates [93–96].
Drosophila septate junctions
Invertebrates often show septate junctions that morphologically resemble those found in the paranodal regions at the nodes of Ranvier [34, 72]. Within the nervous system, they are prominently formed between subperineurial glial cells. A hallmark of all septate junctions is the NeurexinIV protein, which is the fly homolog of Caspr [97, 98]. In addition, Dcontactin and the Neurofascin155 homolog Neuroglian are localized to septate junctions. At least 15 other components localizing to the septate junctions are known (Table 1). In most cases, loss of any septate junction component results in the disassembly of morphologically discernable septate junctions [40, 99]. Based on the large number of proteins that are required to build functional septate junctions, it is hard to imagine how they assemble the elaborate ladder-like structures that are recognized in the TEM. Questions concerning the stability of junctions and their remodeling during epithelia morphogenesis are currently unclear and need further research. Likewise, it is not known how a possible paracellular transport of small molecules can be controled by septate junctions. Possibly, the difference between flies, where septate junctions define the paracellular seal, and the septate-like junctions of paranodes in vertebrates, which do not provide such a tight sealing function, resides in the integration of Claudin-like proteins in the Drosophila septate junctions [24]. One interesting component of the septate junctions in Drosophila is the Nervana2 (Nrv2) protein. Interestingly, Nrv2 was reported to be a component of septate junctions found in epithelial cells and nrv2 mutants show leaky trachea and a defective blood–brain barrier (BBB) [24, 100–102]. The function of Nrv2 in cell adhesion seems to be pump-independent, as it is already described for Na/K-ATPases in the vertebrate system [100]. Interestingly, Nrv2 is strongly expressed in the wrapping glial cells, which do not appear to form septate junctions [24].
A clear hint that septate junctions control paracellular transport stems from the analysis of the gene moody. Mutations in the gene moody have been identified in a forward genetic screen for Drosophila mutants with altered cocaine sensitivity [36]. Moody mutants have abnormal subperineurial cells, which exhibit septate junctions of reduced length [33]. In consequence, the BBB is leaky and fluorescently labeled dextrane penetrates into the nerve cord [33]. Within the nervous system, only the subperineurial glial cells continuously express the GPCR Moody. Interestingly, transient RNAi expression leads to a temporary and reversible breakdown of the BBB [33]. Thus, the GPCR Moody is required for both the establishment and the maintenance of the BBB. Possibly, Moody regulates the actin cytoskeleton, which has been demonstrated to have a pivotal function in the peripheral glia [103], to position the different components of the septate junctions.
Surprisingly, however, flies lacking all moody function are viable and can be easily kept as homozygous stock. GPCRs transmit signals through trimeric G-proteins, inducing a guanine nucleotide exchange on their Gα-subunits [104]. A role for G-protein signaling in Drosophila BBB formation was first suggested by the identification of the gene loco, which encodes an RGS-type protein expressed in a larger subset of glial cells [105]. Although loco mutants have been studied for a long time, the precise integration of Loco function into glial G-protein signaling has not yet been elucidated.
Control of septate junction formation via mRNA splicing
The septate junctions form relatively late at the very end of embryonic development [34]. Genetic screens have identified two mRNA processing factors as being essential for septate junction formation. crooked neck (crn) was shown to be required for the correct splicing of the neurexinIV pre-mRNA [106]. The gene how encodes three different mRNA binding proteins [HOW(S), HOW(M) and HOW(L)] which are linked to mRNA stability and splicing. Crn is an essential and evolutionary well-conserved general splicing factor, which binds to HOW(S) in the cytoplasm [106–108]. The HOW proteins are members of the STAR family (Signal Transduction and Activation of RNA) of RNA-binding proteins [109]. The STAR proteins are evolutionary well conserved and Quaking and Sam68 are well-known homologs in mammals [110, 111]. The Crn/HOW(S) complex must then be shuttled into the nucleus to modulate the splicing of neurexinIV and nervana2, which are both essential septate junction components [98, 101, 102, 106]. The neurexinIV gene generates two different isoforms by mutually exclusive and “tissue-specific” usage of exons 3 and 4. The protein encoded by the neurexinIV Exon3 mRNA is enriched in septate junction-forming tissues and interacts with Dcontactin and Neuroglian, whereas the protein encoded by the neurexinIV Exon4 mRNA is expressed by axons where it binds to the Ig domain protein Wrapper, which is expressed on the midline glia. This binding does not result in the formation of septate junctions [112–114].
What could be the benefit of a splicing-related mechanism in controlling septate junction formation? Possibly, in an initial phase, both NeurexinIV isoforms are generated and only upon a differentiation-dependent signal, e.g., when septate junctions reach certain maturity, is the nuclear import of the Crn/HOW(S) complex initiated and expression of the NeurexinIVExon3 isoform dominates. The exact molecular mechanism underlying this tissue-specific splicing is still unknown. Members of the STAR protein family, such as HOW, are often expressed tissue-specifically and have the ability to undergo post-transcriptional modification such as phosphorylation [115–118]. In humans, for example, a signal-dependent regulation of splicing can be regulated by phosphorylation of Sam68, which modulates the inclusion of exon-v5 of the CD44 mRNA [115]. A similar mechanism may operate for HOW function.
Conclusions and outlook
Here, we have shown that the overall organization of peripheral nerves of vertebrates is akin to the one of peripheral nerves in Drosophila. Both on a morphological and on a molecular level, astonishing homologies have been revealed in the past years. Wrapping of axons is regulated in very similar ways in Drosophila and mammals—despite the fact that in our nervous system myelin predominates. The apparent conservation of basic cellular mechanisms will very likely promote the analysis of genes and proteins first identified in mammalian myelin. The myelin proteome has been determined and long lists of proteins exist, which now need to be tested for their biological relevance [119–121]. A large fraction of the identified proteins have clear homologs in Drosophila and their relevance for glial differentiation is now being assayed using cell-type-specific RNA interference technologies (Table 2; [122–125]). In fact, for some of the fly homologs of the 294 proteins of the myelin proteome, glial expression has been noted [126, 127]. The regulation of septate junction formation by splicing and possibly post-translational control of the nuclear access of splicing factors appears also evolutionary conserved. The HOW homolog Quaking is needed for proper development of myelinated nerves and has also been linked to the control of splicing [128–131]. This suggests that elucidation of the mechanism underlying the action of the Crn/HOW complex will shed some light on the biology of myelination.
Table 2.
Drosophila homologs of the vertebrate mylein proteom
| Vertebrate ID | Vertebrates symbol | Drosophila CG | Drosophila symbol |
|---|---|---|---|
| P35762/P40240/Q922J6/P40237 | Cd81/Cd9/Tspan2/Cd82 | CG6120 | Tsp96F |
| Q8VDN2/Q6PIE5/Q9WV27 | Atp1a1/2/4 | CG5670 | Atp α* |
| P56371/Q91ZR1 | Rab4a/4b | CG4921 | Rab4 |
| P62835/Q99JI6 | Rap1a/b | CG1956 | R |
| P07901/P11499 | Hsp90aa1/ab1 | CG1242 | Hsp83* |
| Q7TQD2/Q9CRB6 | Tppp/Tppp3 | CG4893 | CG4893 |
| P16388/P63141/P16390 | Kcna1/2/3 | CG12348 | Sh |
| B2RSH2/P08752/Q9DC51 | Gnai1/2/3 | CG10060 | G-i 65A |
| O35526/P61264 | Stx1a/b | CG31136 | Syx1A |
| P26040/P26041/P26043 | Ezr/Msn/Rdx | CG10701 | Moe |
| P63321/Q9JIW9 | Rala/b | CG2849 | Rala |
| P21278/P21279 | Gna11/q | CG17759 | G 49B |
| P50396/Q61598 | Gdi1/2 | CG4422 | Gdi* |
| Q9CQV8/P63101 | Ywhab/z | CG17870 | 14-3-3zeta* |
| P35803/P60202 | Gpm6b/Plp1 | CG7540 | M6* |
| P63011/P62823 | Rab3a/c | CG7576 | Rab3 |
| Q504M8/Q9JKM7 | Rab26/37 | CG34410 | Rab26 |
| Q7TMM9/P68372/Q9D6F9/P99024 | Tubb2a/2c/4/5 | CG9359 | βTub85D |
| P62874/P62880/P29387 | Gnb1/2/4 | CG10545 | G13F |
| P06151/P16125/P00342 | Ldha/b/c | CG10160 | ImpL3 |
| P17182/P17183/P21550 | Eno1/2/3 | CG17654 | Eno |
| P68369/P05213/P68373/P05214 | Tuba1a/1b/1c/3a | CG1913; CG2512 | αTub84B; αTub84D |
| P15532/Q01768 | Nme1/2 | CG2210 | Awd |
| P09411/P09041 | Pgk1/2 | CG3127 | Pgk |
| P62821/Q9D1G1 | Rab1/1b | CG3320 | Rab1 |
| O88935/Q64332 | Syn1/2 | CG3985 | Syn |
| Q8CHH9/Q8C1B7 | Sep8/11 | CG4173; CG2916 | Sep2; Sep5 |
| P45591/Q9R0P5 | Cfl2/Dstn | CG4254; CG6873 | Tsr; CG6873 |
| P05064/P05063 | Aldoa/c | CG6058 | Ald |
| Q9WVK4/Q9QXY6/Q9EQP2 | Ehd1/3/4 | CG6148 | Past1 |
| Q9DB05/P28663 | Napa/b | CG6625 | Snap |
| Q91X97/P62748 | Ncald/Hpcal1 | CG7641 | Nca |
| P10126/P62631 | Eef1a1/2 | CG8280; CG1873 | Ef1α48D; Ef1α100E |
| P12960 | Cntn1 | CG1084 | Cont |
| Q8R464 | Cadm4 | CG10095; CG12591 | dpr15; dpr16 |
| P13595 | Ncam1 | CG3665 | Fas2* |
| Q63912 | Omg | CG42709 | CG42709 |
| Q8VDQ8 | Sirt2 | CG5085 | Sirt2 |
| P62259 | Ywhae | CG31196 | 14-3-3ε |
| P60710 | Actb | CG12051 | Act42A |
| P40124 | Cap1 | CG33979 | Capt |
| P84078 | Arf1 | CG8385 | Arf79F |
| P84084 | Arf5 | CG11027 | Arf102F* |
| P62331 | Arf6 | CG8156 | Arf51F |
| O08539 | Bin1 | CG8604 | Amph |
| Q8K298 | Anln | CG2092 | Scra |
| P16460 | Ass1 | CG1315 | CG1315 |
| Q0VF55 | Atp2b3 | CG42314 | PMCA |
| P62204 | Calm3 | CG8472 | Cam* |
| P35564 | Canx | CG9906; CG11958; CG1924 | CG9906; Cnx99A; CG1924 |
| Q923T9 | Camk2 g | CG18069 | CaMKII |
| P00920 | Car2 | CG7820 | CAH1 |
| P61164 | Actr1a | CG6174 | Arp87C |
| O54991 | Cntnap1 | CG6827 | Nrx-IV |
| Q9WUM4 | Coro1c | CG9446 | Coro |
| Q04447 | Ckb | CG32031; CG5144 | Argk*, **; CG5144 |
| P23927 | Cryab | CG4533 | l(2)efl |
| O08553 | Dpysl2 | CG1411 | CRMP |
| Q99KJ8 | Dctn2 | CG8269 | Dmn |
| Q9JHU4 | Dync1h1 | CG7507 | Dhc64C |
| O70251 | Eef1b2 | CG6341 | Ef1β |
| P58252 | Eef2 | CG2238 | Ef2b |
| P19096 | Fasn | CG3524; CG3523 | v(2)k05816; CG3523 |
| O08917 | Flot1 | CG8200 | Flo* |
| P62881 | Gnb5 | CG10763 | Gβ5 |
| Q9DAS9 | Gng12 | CG8261 | Gγ1 |
| P16858 | Gapdh | CG12055; CG8893 | Gapdh1; Gapdh2* |
| P13020 | Gsn | CG1106 | Gel |
| P06745 | Gpi1 | CG8251 | Pgi |
| P05201 | Got1 | CG8430 | Got1* |
| P56564 | Slc1a3 | CG3747 | Eaat1** |
| P15105 | Glul | CG1743 | Gs2*,** |
| P62827 | Ran | CG1404 | ran |
| Q61316 | Hspa4 | CG6603 | Hsc70Cb |
| P20029 | Hspa5 | CG4147 | Hsc70-3 |
| P63017 | Hspa8 | CG4264 | Hsc70-4 |
| P62482 | Kcnab2 | CG32688 | Hk |
| Q9D1G5 | Lrrc57 | CG3040 | CG3040 |
| Q8BLK3 | Lsamp | CG12369; CG2198 | Lac*,**; Ama* |
| P14152 | Mdh1 | CG5362 | CG5362 |
| P14873 | Mtap1b | CG34387 | futsch |
| P63085 | Mapk1 | CG12559 | rl |
| Q5SYD0 | Myo1d | CG7438 | Myo31DF |
| P14094 | Atp1b1 | CG9261; CG9258; CG8663 |
nrv2*,**; nrv1; nrv3 |
| P55012 | Slc12a2 | CG4357 | Ncc69 |
| P46460 | Nsf | CG33101; CG1618 | Nsf2; comt |
| Q99K10 | Nlgn1 | CG13772 | neuroligin |
| Q62433 | Ndrg1 | CG15669 | MESK2 |
| Q99LX0 | Park7 | CG1349; CG6646 | dj-1β; DJ-1α |
| P99029 | Prdx5 | CG7217 | Prx5 |
| P53810 | Pitpna | CG5269 | Vib |
| P47857 | Pfkm | CG4001 | Pfk |
| Q61753 | Phgdh | CG6287 | CG6287 |
| Q9DBJ1 | Pgam1 | CG1721; CG17645 | Pglym78; Pglym87 |
| Q9Z1B3 | Plcb1 | CG4574 | Plc21C |
| Q99K85 | Psat1 | CG11899 | CG11899 |
| P67778 | Phb | CG10691 | l(2)37Cc |
| O35129 | Phb2 | CG15081 | l(2)03709* |
| P27773 | Pdia3 | CG8983 | ERp60* |
| P63318 | Prkcc | CG6622 | Pkc53E |
| P52480 | Pkm2 | CG7070 | PyK |
| Q8BVI4 | Qdpr | CG4665 | Dhpr |
| P53994 | Rab2a | CG3269 | Rab2 |
| P51150 | Rab7 | CG5915 | Rab7* |
| P61028 | Rab8b | CG8287 | Rab8 |
| P61027 | Rab10 | CG17060 | Rab10 |
| Q91V41 | Rab14 | CG4212 | Rab14 |
| P35293 | Rab18 | CG3129 | Rab-RP4* |
| Q923S9 | Rab30 | CG9100 | Rab30 |
| Q8BHC1 | Rab39b | CG12156 | Rab39 |
| Q8CG50 | Rab43 | CG7062 | Rab-RP3 |
| P60764 | Rac3 | CG2248; CG8556 | Rac1; Rac2** |
| Q80ZJ1 | Rap2a | CG3204 | Rap2 l |
| Q99PT1 | Arhgdia | CG7823 | RhoGDI |
| Q9QUI0 | Rhoa | CG8416 | Rho1 |
| P42208 | Sep1 | CG1403 | Sep1 |
| Q91V61 | Sfxn3 | CG11739 | CG11739 |
| Q9CWZ7 | Napg | CG3988; CG6208 | γSnap; CG6208 |
| Q62261 | Spnb2 | CG5870 | β-Spec* |
| Q60864 | Stip1 | CG2720 | Hop |
| P08228 | Sod1 | CG11793 | Sod* |
| P60879 | Snap25 | CG9474; CG40452 | Snap24; Snap25 |
| P46096 | Syt1 | CG3139 | Syt1 |
| Q61644 | Pacsin1 | CG33094 | Synd |
| P11983 | Tcp1 | CG5374 | T-cp1 |
| P80314 | Cct2 | CG7033 | CG7033* |
| P80315 | Cct4 | CG5525 | CG5525 |
| P80316 | Cct5 | CG8439 | Cct5 |
| P80318 | Cct3 | CG8977 | Cctγ |
| Q9R1Q8 | Tagln3 | CG14996; CG5023 | Chd64; CG5023 |
| Q01853 | Vcp | CG2331 | TER94 |
| P40142 | Tkt | CG8036; CG5103 | CG8036; CG5103 |
| P17751 | Tpi1 | CG2171 | Tpi |
| P62991 | Ub | CG5271; CG2960; CG11624 |
RpS27A; RpL40; Ubi-p63E |
| Q02053 | Uba1 | CG1782 | Uba1* |
| Q9R0P9 | Uchl1 | CG4265 | Uch |
| P50516 | Atp6v1a | CG3762; CG12403 | Vha68-2; Vha68-1 |
| P62814 | Atp6v1b2 | CG17369 | Vha55 |
| Q9Z1G3 | Atp6v1c1 | CG8048 | Vha44 |
| P50518 | Atp6v1e1 | CG1088 | Vha26 |
| O88342 | Wdr1 | CG10724 | Flr |
The left hand column lists the myelin proteom according to Jahn et al. (2009). Only proteins with Drosophila homologs are shown. Drosophila proteins marked with a * were already identified as being expressed in glial cells by Altenhein et al. [127] and proteins marked with ** were identified by Freeman et al. [126]
Acknowledgments
We are thankful for much help from members of the laboratory for support throughout the work. The laboratory has received funding from the European Community’s Seventh Framework Programme (FP7/2007-2013) under grant agreement No. HEALTH-F2-2008-201535.
References
- 1.Nave KA. Myelination and the trophic support of long axons. Nat Rev Neurosci. 2010;11:275–283. doi: 10.1038/nrn2797. [DOI] [PubMed] [Google Scholar]
- 2.Woodhoo A, Sommer L. Development of the Schwann cell lineage: from the neural crest to the myelinated nerve. Glia. 2008;56:1481–1490. doi: 10.1002/glia.20723. [DOI] [PubMed] [Google Scholar]
- 3.Le Douarin NM, Dupin E. Multipotentiality of the neural crest. Curr Opin Genet Dev. 2003;13:529–536. doi: 10.1016/j.gde.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Le Douarin NM, Creuzet S, Couly G, Dupin E. Neural crest cell plasticity and its limits. Development. 2004;131:4637–4650. doi: 10.1242/dev.01350. [DOI] [PubMed] [Google Scholar]
- 5.Nave KA, Trapp BD. Axon-glial signaling and the glial support of axon function. Annu Rev Neurosci. 2008;31:535–561. doi: 10.1146/annurev.neuro.30.051606.094309. [DOI] [PubMed] [Google Scholar]
- 6.Haller FR, Low FN. The fine structure of the peripheral nerve root sheath in the subarachnoid space in the rat and other laboratory animals. Am J Anat. 1971;131:1–19. doi: 10.1002/aja.1001310102. [DOI] [PubMed] [Google Scholar]
- 7.Kristensson K, Olsson Y. The perineurium as a diffusion barrier to protein tracers. Differences between mature and immature animals. Acta Neuropathol. 1971;17:127–138. doi: 10.1007/BF00687488. [DOI] [PubMed] [Google Scholar]
- 8.Allt G. Ultrastructural features of the immature peripheral nerve. J Anat. 1969;105:283–293. [PMC free article] [PubMed] [Google Scholar]
- 9.Jessen KR, Mirsky R, Salzer J. Introduction. Schwann cell biology. Glia. 2008;56:1479–1480. doi: 10.1002/glia.20779. [DOI] [PubMed] [Google Scholar]
- 10.Grim M, Halata Z, Franz T. Schwann cells are not required for guidance of motor nerves in the hindlimb in Splotch mutant mouse embryos. Anat Embryol (Berl) 1992;186:311–318. doi: 10.1007/BF00185979. [DOI] [PubMed] [Google Scholar]
- 11.Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6:671–682. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi M, Osumi N. Identification of a novel type II classical cadherin: rat cadherin19 is expressed in the cranial ganglia and Schwann cell precursors during development. Dev Dyn. 2005;232:200–208. doi: 10.1002/dvdy.20209. [DOI] [PubMed] [Google Scholar]
- 13.Taveggia C, Zanazzi G, Petrylak A, Yano H, Rosenbluth J, Einheber S, Xu X, Esper RM, Loeb JA, Shrager P, Chao MV, Falls DL, Role L, Salzer JL. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47:681–694. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michailov GV, Sereda MW, Brinkmann BG, Fischer TM, Haug B, Birchmeier C, Role L, Lai C, Schwab MH, Nave KA. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304:700–703. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- 15.Chen S, Velardez MO, Warot X, Yu ZX, Miller SJ, Cros D, Corfas G. Neuregulin 1-erbB signaling is necessary for normal myelination and sensory function. J Neurosci. 2006;26:3079–3086. doi: 10.1523/JNEUROSCI.3785-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du Plessis DG, Mouton YM, Muller CJ, Geiger DH. An ultrastructural study of the development of the chicken perineurial sheath. J Anat. 1996;189(Pt 3):631–641. [PMC free article] [PubMed] [Google Scholar]
- 17.Kucenas S, Takada N, Park HC, Woodruff E, Broadie K, Appel B. CNS-derived glia ensheath peripheral nerves and mediate motor root development. Nat Neurosci. 2008;11:143–151. doi: 10.1038/nn2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Hilchen CM, Beckervordersandforth RM, Rickert C, Technau GM, Altenhein B. Identity, origin, and migration of peripheral glial cells in the Drosophila embryo. Mech Dev. 2008;125:337–352. doi: 10.1016/j.mod.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Sepp KJ, Schulte J, Auld VJ. Developmental dynamics of peripheral glia in Drosophila melanogaster. Glia. 2000;30:122–133. doi: 10.1002/(SICI)1098-1136(200004)30:2<122::AID-GLIA2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 20.Sink H, Whitington PM. Location and connectivity of abdominal motoneurons in the embryo and larva of Drosophila melanogaster. J Neurobiol. 1991;22:298–311. doi: 10.1002/neu.480220309. [DOI] [PubMed] [Google Scholar]
- 21.Mahr A, Aberle H. The expression pattern of the Drosophila vesicular glutamate transporter: a marker protein for motoneurons and glutamatergic centers in the brain. Gene Expr Patterns. 2006;6:299–309. doi: 10.1016/j.modgep.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 22.Ghysen A, Dambly-Chaudiere C, Aeeves E, Jan LY, Jan YN. Sensory neurons and peripheral pathways in Drosophila embryos. Roux’s Arch Dev Biol. 1986;195:281–289. doi: 10.1007/BF00376060. [DOI] [PubMed] [Google Scholar]
- 23.Bodmer R, Carretto R, Jan YN. Neurogenesis of the peripheral nervous system in Drosophila embryos: DNA replication patterns and cell lineages. Neuron. 1989;3:21–32. doi: 10.1016/0896-6273(89)90112-8. [DOI] [PubMed] [Google Scholar]
- 24.Stork T, Engelen D, Krudewig A, Silies M, Bainton RJ, Klambt C. Organization and function of the blood-brain barrier in Drosophila . J Neurosci. 2008;28:587–597. doi: 10.1523/JNEUROSCI.4367-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olofsson B, Page DT. Condensation of the central nervous system in embryonic Drosophila is inhibited by blocking hemocyte migration or neural activity. Dev Biol. 2005;279:233–243. doi: 10.1016/j.ydbio.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 26.Sun B, Xu P, Salvaterra PM. Dynamic visualization of nervous system in live Drosophila . Proc Natl Acad Sci USA. 1999;96:10438–10443. doi: 10.1073/pnas.96.18.10438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun B, Salvaterra PM. Two Drosophila nervous system antigens, Nervana 1 and 2, are homologous to the beta subunit of Na+, K(+)-ATPase. Proc Natl Acad Sci USA. 1995;92:5396–5400. doi: 10.1073/pnas.92.12.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun B, Wang W, Salvaterra PM. Functional analysis and tissue-specific expression of Drosophila Na+, K+-ATPase subunits. J Neurochem. 1998;71:142–151. doi: 10.1046/j.1471-4159.1998.71010142.x. [DOI] [PubMed] [Google Scholar]
- 29.Sun B, Xu P, Wang W, Salvaterra PM. In vivo modification of Na(+), K(+)-ATPase activity in Drosophila . Comp Biochem Physiol B Biochem Mol Biol. 2001;130:521–536. doi: 10.1016/S1096-4959(01)00470-5. [DOI] [PubMed] [Google Scholar]
- 30.Pereanu W, Shy D, Hartenstein V. Morphogenesis and proliferation of the larval brain glia in Drosophila . Dev Biol. 2005;283:191–203. doi: 10.1016/j.ydbio.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 31.Morin X, Daneman R, Zavortink M, Chia W. A protein trap strategy to detect GFP-tagged proteins expressed from their endogenous loci in Drosophila . Proc Natl Acad Sci USA. 2001;98:15050–15055. doi: 10.1073/pnas.261408198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jia XX, Gorczyca M, Budnik V. Ultrastructure of neuromuscular junctions in Drosophila: comparison of wild type and mutants with increased excitability. J Neurobiol. 1993;24:1025–1044. doi: 10.1002/neu.480240804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwabe T, Bainton RJ, Fetter RD, Heberlein U, Gaul U. GPCR signaling is required for blood-brain barrier formation in Drosophila . Cell. 2005;123:133–144. doi: 10.1016/j.cell.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 34.Tepass U, Hartenstein V. The development of cellular junctions in the Drosophila embryo. Dev Biol. 1994;161:563–596. doi: 10.1006/dbio.1994.1054. [DOI] [PubMed] [Google Scholar]
- 35.Mayer F, Mayer N, Chinn L, Pinsonneault RL, Kroetz D, Bainton RJ. Evolutionary conservation of vertebrate blood-brain barrier chemoprotective mechanisms in Drosophila . J Neurosci. 2009;29:3538–3550. doi: 10.1523/JNEUROSCI.5564-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bainton RJ, Tsai LT, Schwabe T, DeSalvo M, Gaul U, Heberlein U. Moody encodes two GPCRs that regulate cocaine behaviors and blood-brain barrier permeability in Drosophila . Cell. 2005;123:145–156. doi: 10.1016/j.cell.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 37.Sepp KJ, Auld VJ. Conversion of lacZ enhancer trap lines to GAL4 lines using targeted transposition in Drosophila melanogaster . Genetics. 1999;151:1093–1101. doi: 10.1093/genetics/151.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schulte J, Tepass U, Auld VJ. Gliotactin, a novel marker of tricellular junctions, is necessary for septate junction development in Drosophila . J Cell Biol. 2003;161:991–1000. doi: 10.1083/jcb.200303192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laprise P, Beronja S, Silva-Gagliardi NF, Pellikka M, Jensen AM, McGlade CJ, Tepass U. The FERM protein Yurt is a negative regulatory component of the Crumbs complex that controls epithelial polarity and apical membrane size. Dev Cell. 2006;11:363–374. doi: 10.1016/j.devcel.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laprise P, Lau KM, Harris KP, Silva-Gagliardi NF, Paul SM, Beronja S, Beitel GJ, McGlade CJ, Tepass U. Yurt, Coracle, Neurexin IV and the Na(+), K(+)-ATPase form a novel group of epithelial polarity proteins. Nature. 2009;459:1141–1145. doi: 10.1038/nature08067. [DOI] [PubMed] [Google Scholar]
- 41.Laprise P, Paul SM, Boulanger J, Robbins RM, Beitel GJ, Tepass U. Epithelial polarity proteins regulate Drosophila tracheal tube size in parallel to the luminal matrix pathway. Curr Biol. 2010;20:55–61. doi: 10.1016/j.cub.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leiserson WM, Harkins EW, Keshishian H. Fray, a Drosophila serine/threonine kinase homologous to mammalian PASK, is required for axonal ensheathment. Neuron. 2000;28:793–806. doi: 10.1016/S0896-6273(00)00154-9. [DOI] [PubMed] [Google Scholar]
- 43.Gagnon KB, England R, Delpire E. Characterization of SPAK and OSR1, regulatory kinases of the Na-K-2Cl cotransporter. Mol Cell Biol. 2006;26:689–698. doi: 10.1128/MCB.26.2.689-698.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lavery W, Hall V, Yager JC, Rottgers A, Wells MC, Stern M. Phosphatidylinositol 3-kinase and Akt nonautonomously promote perineurial glial growth in Drosophila peripheral nerves. J Neurosci. 2007;27:279–288. doi: 10.1523/JNEUROSCI.3370-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Awasaki T, Lai SL, Ito K, Lee T. Organization and postembryonic development of glial cells in the adult central brain of Drosophila . J Neurosci. 2008;28:13742–13753. doi: 10.1523/JNEUROSCI.4844-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Curtin KD, Wyman RJ, Meinertzhagen IA. Basigin/EMMPRIN/CD147 mediates neuron-glia interactions in the optic lamina of Drosophila . Glia. 2007;55:1542–1553. doi: 10.1002/glia.20568. [DOI] [PubMed] [Google Scholar]
- 47.Curtin KD, Meinertzhagen IA, Wyman RJ. Basigin (EMMPRIN/CD147) interacts with integrin to affect cellular architecture. J Cell Sci. 2005;118:2649–2660. doi: 10.1242/jcs.02408. [DOI] [PubMed] [Google Scholar]
- 48.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maurel P, Salzer JL. Axonal regulation of Schwann cell proliferation and survival and the initial events of myelination requires PI 3-kinase activity. J Neurosci. 2000;20:4635–4645. doi: 10.1523/JNEUROSCI.20-12-04635.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Monk KR, Naylor SG, Glenn TD, Mercurio S, Perlin JR, Dominguez C, Moens CB, Talbot WS. A G protein-coupled receptor is essential for Schwann cells to initiate myelination. Science. 2009;325:1402–1405. doi: 10.1126/science.1173474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Monk KR, Talbot WS. Genetic dissection of myelinated axons in zebrafish. Curr Opin Neurobiol. 2009;19:486–490. doi: 10.1016/j.conb.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Franzdottir SR, Engelen D, Yuva-Aydemir Y, Schmidt I, Aho A, Klambt C. Switch in FGF signalling initiates glial differentiation in the Drosophila eye. Nature. 2009;460:758–761. doi: 10.1038/nature08167. [DOI] [PubMed] [Google Scholar]
- 53.Silies M, Yuva-Aydemir Y, Franzdottir SR, Klämbt C. The eye imaginal disc as a model to study the coordination of neuronal and glial development. Fly (Austin) 2010;4:71–79. doi: 10.4161/fly.4.1.11312. [DOI] [PubMed] [Google Scholar]
- 54.Wolff T, Ready DF. Pattern formation in the Drosophila retina. In: Bate M, Martinez Arias A, editors. The development of Drosophila. Cold Spring Harbor: Cold Spring Harbor Press; 1993. pp. 1277–1325. [Google Scholar]
- 55.Rangarajan R, Courvoisier H, Gaul U. Dpp and Hedgehog mediate neuron-glia interactions in Drosophila eye development by promoting the proliferation and motility of subretinal glia. Mech Dev. 2001;108:93–103. doi: 10.1016/S0925-4773(01)00501-9. [DOI] [PubMed] [Google Scholar]
- 56.Silies M, Yuva Y, Engelen D, Aho A, Stork T, Klambt C. Glial cell migration in the eye disc. J Neurosci. 2007;27:13130–13139. doi: 10.1523/JNEUROSCI.3583-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rangarajan R, Gong Q, Gaul U. Migration and function of glia in the developing Drosophila eye. Development. 1999;126:3285–3292. doi: 10.1242/dev.126.15.3285. [DOI] [PubMed] [Google Scholar]
- 58.Hummel T, Attix S, Gunning D, Zipursky SL. Temporal control of glial cell migration in the Drosophila eye requires gilgamesh, hedgehog, and eye specification genes. Neuron. 2002;33:193–203. doi: 10.1016/S0896-6273(01)00581-5. [DOI] [PubMed] [Google Scholar]
- 59.Choi KW, Benzer S. Migration of glia along photoreceptor axons in the developing Drosophila eye. Neuron. 1994;12:423–431. doi: 10.1016/0896-6273(94)90282-8. [DOI] [PubMed] [Google Scholar]
- 60.Roots BI, Lane NJ. Myelinating glia of earthworm giant axons: thermally induced intramembranous changes. Tissue Cell. 1983;15:695–709. doi: 10.1016/0040-8166(83)90044-7. [DOI] [PubMed] [Google Scholar]
- 61.Gunther J. Impulse conduction in the myelinated giant fibers of the earthworm. Structure and function of the dorsal nodes in the median giant fiber. J Comp Neurol. 1976;168:505–531. doi: 10.1002/cne.901680405. [DOI] [PubMed] [Google Scholar]
- 62.Weatherby TM, Davis AD, Hartline DK, Lenz PH. The need for speed. II. Myelin in calanoid copepods. J Comp Physiol [A] 2000;186:347–357. doi: 10.1007/s003590050435. [DOI] [PubMed] [Google Scholar]
- 63.Lenz PH, Hartline DK, Davis AD. The need for speed. I. Fast reactions and myelinated axons in copepods. J Comp Physiol [A] 2000;186:337–345. doi: 10.1007/s003590050434. [DOI] [PubMed] [Google Scholar]
- 64.Davis AD, Weatherby TM, Hartline DK, Lenz PH. Myelin-like sheaths in copepod axons. Nature. 1999;398:571. doi: 10.1038/19212. [DOI] [PubMed] [Google Scholar]
- 65.Govind CK, Pearce J. Remodeling of nerves during claw reversal in adult snapping shrimps. J Comp Neurol. 1988;268:121–130. doi: 10.1002/cne.902680112. [DOI] [PubMed] [Google Scholar]
- 66.Heuser JE, Doggenweiler CF. The fine structural organization of nerve fibers, sheaths, and glial cells in the prawn, Palaemonetes vulgaris . J Cell Biol. 1966;30:381–403. doi: 10.1083/jcb.30.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu K, Terakawa S. Fenestration nodes and the wide submyelinic space form the basis for the unusually fast impulse conduction of shrimp myelinated axons. J Exp Biol. 1999;202:1979–1989. doi: 10.1242/jeb.202.15.1979. [DOI] [PubMed] [Google Scholar]
- 68.Hama K. The fine structure of the Schwann cell sheath of the nerve fiber in the shrimp (Penaeus japonicus) J Cell Biol. 1966;31:624–632. doi: 10.1083/jcb.31.3.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Johnston WL, Dyer JR, Castellucci VF, Dunn RJ. Clustered voltage-gated Na + channels in Aplysia axons. J Neurosci. 1996;16:1730–1739. doi: 10.1523/JNEUROSCI.16-05-01730.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Borner J, Puschmann T, Duch C. A steroid hormone affects sodium channel expression in Manduca central neurons. Cell Tissue Res. 2006;325:175–187. doi: 10.1007/s00441-006-0175-7. [DOI] [PubMed] [Google Scholar]
- 71.Poliak S, Peles E. The local differentiation of myelinated axons at nodes of Ranvier. Nat Rev Neurosci. 2003;4:968–980. doi: 10.1038/nrn1253. [DOI] [PubMed] [Google Scholar]
- 72.Salzer JL, Brophy PJ, Peles E. Molecular domains of myelinated axons in the peripheral nervous system. Glia. 2008;56:1532–1540. doi: 10.1002/glia.20750. [DOI] [PubMed] [Google Scholar]
- 73.Schafer DP, Rasband MN. Glial regulation of the axonal membrane at nodes of Ranvier. Curr Opin Neurobiol. 2006;16:508–514. doi: 10.1016/j.conb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 74.Susuki K, Rasband MN. Molecular mechanisms of node of Ranvier formation. Curr Opin Cell Biol. 2008;20:616–623. doi: 10.1016/j.ceb.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.MacKenzie ML, Ghabriel MN, Allt G. Nodes of Ranvier and Schmidt-Lanterman incisures: an in vivo lanthanum tracer study. J Neurocytol. 1984;13:1043–1055. doi: 10.1007/BF01148601. [DOI] [PubMed] [Google Scholar]
- 76.Hirano A, Becker NH, Zimmerman HM. Isolation of the periaxonal space of the central myelinated nerve fiber with regard to the diffusion of peroxidase. J Histochem Cytochem. 1969;17:512–516. doi: 10.1177/17.8.512. [DOI] [PubMed] [Google Scholar]
- 77.Banerjee S, Bhat MA. Neuron-glial interactions in blood–brain barrier formation. Annu Rev Neurosci. 2007;30:235–258. doi: 10.1146/annurev.neuro.30.051606.094345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bhat MA. Molecular organization of axo-glial junctions. Curr Opin Neurobiol. 2003;13:552–559. doi: 10.1016/j.conb.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 79.Einheber S, Zanazzi G, Ching W, Scherer S, Milner TA, Peles E, Salzer JL. The axonal membrane protein Caspr, a homologue of neurexin IV, is a component of the septate-like paranodal junctions that assemble during myelination. J Cell Biol. 1997;139:1495–1506. doi: 10.1083/jcb.139.6.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bhat MA, Rios JC, Lu Y, Garcia-Fresco GP, Ching W, St Martin M, Li J, Einheber S, Chesler M, Rosenbluth J, Salzer JL, Bellen HJ. Axon-glia interactions and the domain organization of myelinated axons requires neurexin IV/Caspr/Paranodin. Neuron. 2001;30:369–383. doi: 10.1016/S0896-6273(01)00294-X. [DOI] [PubMed] [Google Scholar]
- 81.Bo L, Quarles RH, Fujita N, Bartoszewicz Z, Sato S, Trapp BD. Endocytic depletion of L-MAG from CNS myelin in quaking mice. J Cell Biol. 1995;131:1811–1820. doi: 10.1083/jcb.131.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peles E, Nativ M, Lustig M, Grumet M, Schilling J, Martinez R, Plowman GD, Schlessinger J. Identification of a novel contactin-associated transmembrane receptor with multiple domains implicated in protein–protein interactions. EMBO J. 1997;16:978–988. doi: 10.1093/emboj/16.5.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Faivre-Sarrailh C, Banerjee S, Li J, Hortsch M, Laval M, Bhat MA. Drosophila contactin, a homolog of vertebrate contactin, is required for septate junction organization and paracellular barrier function. Development. 2004;131:4931–4942. doi: 10.1242/dev.01372. [DOI] [PubMed] [Google Scholar]
- 84.Boyle ME, Berglund EO, Murai KK, Weber L, Peles E, Ranscht B. Contactin orchestrates assembly of the septate-like junctions at the paranode in myelinated peripheral nerve. Neuron. 2001;30:385–397. doi: 10.1016/S0896-6273(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 85.Bonnon C, Bel C, Goutebroze L, Maigret B, Girault JA, Faivre-Sarrailh C. PGY repeats and N-glycans govern the trafficking of paranodin and its selective association with contactin and neurofascin-155. Mol Biol Cell. 2007;18:229–241. doi: 10.1091/mbc.E06-06-0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rios JC, Melendez-Vasquez CV, Einheber S, Lustig M, Grumet M, Hemperly J, Peles E, Salzer JL. Contactin-associated protein (Caspr) and contactin form a complex that is targeted to the paranodal junctions during myelination. J Neurosci. 2000;20:8354–8364. doi: 10.1523/JNEUROSCI.20-22-08354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gollan L, Salomon D, Salzer JL, Peles E. Caspr regulates the processing of contactin and inhibits its binding to neurofascin. J Cell Biol. 2003;163:1213–1218. doi: 10.1083/jcb.200309147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Charles P, Tait S, Faivre-Sarrailh C, Barbin G, Gunn-Moore F, Denisenko-Nehrbass N, Guennoc AM, Girault JA, Brophy PJ, Lubetzki C. Neurofascin is a glial receptor for the paranodin/Caspr-contactin axonal complex at the axoglial junction. Curr Biol. 2002;12:217–220. doi: 10.1016/S0960-9822(01)00680-7. [DOI] [PubMed] [Google Scholar]
- 89.Zonta B, Tait S, Melrose S, Anderson H, Harroch S, Higginson J, Sherman DL, Brophy PJ. Glial and neuronal isoforms of Neurofascin have distinct roles in the assembly of nodes of Ranvier in the central nervous system. J Cell Biol. 2008;181:1169–1177. doi: 10.1083/jcb.200712154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sherman DL, Brophy PJ. Mechanisms of axon ensheathment and myelin growth. Nat Rev Neurosci. 2005;6:683–690. doi: 10.1038/nrn1743. [DOI] [PubMed] [Google Scholar]
- 91.Pillai AM, Thaxton C, Pribisko AL, Cheng JG, Dupree JL, Bhat MA. Spatiotemporal ablation of myelinating glia-specific neurofascin (Nfasc NF155) in mice reveals gradual loss of paranodal axoglial junctions and concomitant disorganization of axonal domains. J Neurosci Res. 2009;87:1773–1793. doi: 10.1002/jnr.22015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sousa AD, Bhat MA. Cytoskeletal transition at the paranodes: the Achilles’ heel of myelinated axons. Neuron Glia Biol. 2007;3:169–178. doi: 10.1017/S1740925X07000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jarjour AA, Bull SJ, Almasieh M, Rajasekharan S, Baker KA, Mui J, Antel JP, Di Polo A, Kennedy TE. Maintenance of axo-oligodendroglial paranodal junctions requires DCC and netrin-1. J Neurosci. 2008;28:11003–11014. doi: 10.1523/JNEUROSCI.3285-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ishibashi T, Ding L, Ikenaka K, Inoue Y, Miyado K, Mekada E, Baba H. Tetraspanin protein CD9 is a novel paranodal component regulating paranodal junctional formation. J Neurosci. 2004;24:96–102. doi: 10.1523/JNEUROSCI.1484-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dupree JL, Girault JA, Popko B. Axo-glial interactions regulate the localization of axonal paranodal proteins. J Cell Biol. 1999;147:1145–1152. doi: 10.1083/jcb.147.6.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Honke K, Hirahara Y, Dupree J, Suzuki K, Popko B, Fukushima K, Fukushima J, Nagasawa T, Yoshida N, Wada Y, Taniguchi N. Paranodal junction formation and spermatogenesis require sulfoglycolipids. Proc Natl Acad Sci USA. 2002;99:4227–4232. doi: 10.1073/pnas.032068299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Poliak S, Gollan L, Martinez R, Custer A, Einheber S, Salzer JL, Trimmer JS, Shrager P, Peles E. Caspr2, a new member of the neurexin superfamily, is localized at the juxtaparanodes of myelinated axons and associates with K + channels. Neuron. 1999;24:1037–1047. doi: 10.1016/S0896-6273(00)81049-1. [DOI] [PubMed] [Google Scholar]
- 98.Baumgartner S, Littleton JT, Broadie K, Bhat MA, Harbecke R, Lengyel JA, Chiquet-Ehrismann R, Prokop A, Bellen HJ. A Drosophila neurexin is required for septate junction and blood–nerve barrier formation and function. Cell. 1996;87:1059–1068. doi: 10.1016/S0092-8674(00)81800-0. [DOI] [PubMed] [Google Scholar]
- 99.Wu VM, Beitel GJ. A junctional problem of apical proportions: epithelial tube-size control by septate junctions in the Drosophila tracheal system. Curr Opin Cell Biol. 2004;16:493–499. doi: 10.1016/j.ceb.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 100.Paul SM, Palladino MJ, Beitel GJ. A pump-independent function of the Na, K-ATPase is required for epithelial junction function and tracheal tube-size control. Development. 2007;134:147–155. doi: 10.1242/dev.02710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Paul SM, Ternet M, Salvaterra PM, Beitel GJ. The Na+/K+ATPase is required for septate junction function and epithelial tube-size control in the Drosophila tracheal system. Development. 2003;130:4963–4974. doi: 10.1242/dev.00691. [DOI] [PubMed] [Google Scholar]
- 102.Genova JL, Fehon RG. Neuroglian, Gliotactin, and the Na +/K + ATPase are essential for septate junction function in Drosophila . J Cell Biol. 2003;161:979–989. doi: 10.1083/jcb.200212054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sepp KJ, Auld VJ. RhoA and Rac1 GTPases mediate the dynamic rearrangement of actin in peripheral glia. Development. 2003;130:1825–1835. doi: 10.1242/dev.00413. [DOI] [PubMed] [Google Scholar]
- 104.Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3:639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 105.Granderath S, Stollewerk A, Greig S, Goodman CS, O’Kane CJ, Klambt C. Loco encodes an RGS protein required for Drosophila glial differentiation. Development. 1999;126:1781–1791. doi: 10.1242/dev.126.8.1781. [DOI] [PubMed] [Google Scholar]
- 106.Edenfeld G, Volohonsky G, Krukkert K, Naffin E, Lammel U, Grimm A, Engelen D, Reuveny A, Volk T, Klambt C. The splicing factor crooked neck associates with the RNA-binding protein HOW to control glial cell maturation in Drosophila . Neuron. 2006;52:969–980. doi: 10.1016/j.neuron.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 107.Chung S, McLean MR, Rymond BC. Yeast ortholog of the Drosophila crooked neck protein promotes spliceosome assembly through stable U4/U6.U5 snRNP addition. RNA. 1999;5:1042–1054. doi: 10.1017/S1355838299990635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Burnette JM, Hatton AR, Lopez AJ. Trans-acting factors required for inclusion of regulated exons in the Ultrabithorax mRNAs of Drosophila melanogaster . Genetics. 1999;151:1517–1529. doi: 10.1093/genetics/151.4.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vernet C, Artzt K. STAR, a gene family involved in signal transduction and activation of RNA. Trends Genet. 1997;13:479–484. doi: 10.1016/S0168-9525(97)01269-9. [DOI] [PubMed] [Google Scholar]
- 110.Ebersole TA, Chen Q, Justice MJ, Artzt K. The quaking gene product necessary in embryogenesis and myelination combines features of RNA binding and signal transduction proteins. Nat Genet. 1996;12:260–265. doi: 10.1038/ng0396-260. [DOI] [PubMed] [Google Scholar]
- 111.Wang LL, Richard S, Shaw AS. P62 association with RNA is regulated by tyrosine phosphorylation. J Biol Chem. 1995;270:2010–2013. doi: 10.1074/jbc.270.5.2010. [DOI] [PubMed] [Google Scholar]
- 112.Stork T, Thomas S, Rodrigues F, Silies M, Naffin E, Wenderdel S, Klambt C. Drosophila Neurexin IV stabilizes neuron-glia interactions at the CNS midline by binding to Wrapper. Development. 2009;136:1251–1261. doi: 10.1242/dev.032847. [DOI] [PubMed] [Google Scholar]
- 113.Wheeler SR, Banerjee S, Blauth K, Rogers SL, Bhat MA, Crews ST. Neurexin IV and Wrapper interactions mediate Drosophila midline glial migration and axonal ensheathment. Development. 2009;136:1147–1157. doi: 10.1242/dev.030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Noordermeer JN, Kopczynski CC, Fetter RD, Bland KS, Chen WY, Goodman CS. Wrapper, a novel member of the Ig superfamily, is expressed by midline glia and is required for them to ensheath commissural axons in Drosophila . Neuron. 1998;21:991–1001. doi: 10.1016/S0896-6273(00)80618-2. [DOI] [PubMed] [Google Scholar]
- 115.Matter N, Herrlich P, Konig H. Signal-dependent regulation of splicing via phosphorylation of Sam68. Nature. 2002;420:691–695. doi: 10.1038/nature01153. [DOI] [PubMed] [Google Scholar]
- 116.Najib S, Martin-Romero C, Gonzalez-Yanes C, Sanchez-Margalet V. Role of Sam68 as an adaptor protein in signal transduction. Cell Mol Life Sci. 2005;62:36–43. doi: 10.1007/s00018-004-4309-3. [DOI] [PubMed] [Google Scholar]
- 117.Lu Z, Ku L, Chen Y, Feng Y. Developmental abnormalities of myelin basic protein expression in fyn knock-out brain reveal a role of Fyn in posttranscriptional regulation. J Biol Chem. 2005;280:389–395. doi: 10.1074/jbc.M405973200. [DOI] [PubMed] [Google Scholar]
- 118.Zhang Y, Lu Z, Ku L, Chen Y, Wang H, Feng Y. Tyrosine phosphorylation of QKI mediates developmental signals to regulate mRNA metabolism. EMBO J. 2003;22:1801–1810. doi: 10.1093/emboj/cdg171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ishii A, Dutta R, Wark GM, Hwang SI, Han DK, Trapp BD, Pfeiffer SE, Bansal R. Human myelin proteome and comparative analysis with mouse myelin. Proc Natl Acad Sci USA. 2009;106:14605–14610. doi: 10.1073/pnas.0905936106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jahn O, Tenzer S, Werner HB. Myelin proteomics: molecular anatomy of an insulating sheath. Mol Neurobiol. 2009;40:55–72. doi: 10.1007/s12035-009-8071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Taylor CM, Marta CB, Claycomb RJ, Han DK, Rasband MN, Coetzee T, Pfeiffer SE. Proteomic mapping provides powerful insights into functional myelin biology. Proc Natl Acad Sci USA. 2004;101:4643–4648. doi: 10.1073/pnas.0400922101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, Couto A, Marra V, Keleman K, Dickson BJ. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila . Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- 123.O’Brien KP, Remm M, Sonnhammer EL. Inparanoid: a comprehensive database of eukaryotic orthologs. Nucleic Acids Res. 2005;33:D476–D480. doi: 10.1093/nar/gki107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Remm M, Storm CE, Sonnhammer EL. Automatic clustering of orthologs and in-paralogs from pairwise species comparisons. J Mol Biol. 2001;314:1041–1052. doi: 10.1006/jmbi.2000.5197. [DOI] [PubMed] [Google Scholar]
- 125.Berglund AC, Sjolund E, Ostlund G, Sonnhammer EL. InParanoid 6: eukaryotic ortholog clusters with inparalogs. Nucleic Acids Res. 2008;36:D263–D266. doi: 10.1093/nar/gkm1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Freeman MR, Delrow J, Kim J, Johnson E, Doe CQ. Unwrapping glial biology: Gcm target genes regulating glial development, diversification, and function. Neuron. 2003;38:567–580. doi: 10.1016/S0896-6273(03)00289-7. [DOI] [PubMed] [Google Scholar]
- 127.Altenhein B, Becker A, Busold C, Beckmann B, Hoheisel JD, Technau GM. Expression profiling of glial genes during Drosophila embryogenesis. Dev Biol. 2006;296:545–560. doi: 10.1016/j.ydbio.2006.04.460. [DOI] [PubMed] [Google Scholar]
- 128.Larocque D, Fragoso G, Huang J, Mushynski WE, Loignon M, Richard S, Almazan G. The QKI-6 and QKI-7 RNA binding proteins block proliferation and promote Schwann cell myelination. PLoS One. 2009;4:e5867. doi: 10.1371/journal.pone.0005867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Larocque D, Richard S. QUAKING KH domain proteins as regulators of glial cell fate and myelination. RNA Biol. 2005;2:37–40. doi: 10.4161/rna.2.2.1603. [DOI] [PubMed] [Google Scholar]
- 130.Larocque D, Galarneau A, Liu HN, Scott M, Almazan G, Richard S. Protection of p27(Kip1) mRNA by quaking RNA binding proteins promotes oligodendrocyte differentiation. Nat Neurosci. 2005;8:27–33. doi: 10.1038/nn1359. [DOI] [PubMed] [Google Scholar]
- 131.Larocque D, Pilotte J, Chen T, Cloutier F, Massie B, Pedraza L, Couture R, Lasko P, Almazan G, Richard S. Nuclear retention of MBP mRNAs in the quaking viable mice. Neuron. 2002;36:815–829. doi: 10.1016/S0896-6273(02)01055-3. [DOI] [PubMed] [Google Scholar]
- 132.Peles E, Joho K, Plowman GD, Schlessinger J. Close similarity between Drosophila neurexin IV and mammalian Caspr protein suggests a conserved mechanism for cellular interactions. Cell. 1997;88:745–746. doi: 10.1016/S0092-8674(00)81920-0. [DOI] [PubMed] [Google Scholar]
- 133.Gilbert M, Smith J, Roskams AJ, Auld VJ. Neuroligin 3 is a vertebrate gliotactin expressed in the olfactory ensheathing glia, a growth-promoting class of macroglia. Glia. 2001;34:151–164. doi: 10.1002/glia.1050. [DOI] [PubMed] [Google Scholar]
- 134.Charles P, Tait S, Faivre-Sarrailh C, Barbin G, Gunn-Moore F, Denisenko-Nehrbass N, Guennoc A-M, Girault J-A, Brophy PJ, Lubetzki C. Neurofascin is a glial receptor for the paranodin/Caspr-contactin axonal complex at the axoglial junction. Curr Biol. 2002;12:217–220. doi: 10.1016/S0960-9822(01)00680-7. [DOI] [PubMed] [Google Scholar]
- 135.Behr M, Riedel D, Schuh R. The claudin-like megatrachea is essential in septate junctions for the epithelial barrier function in Drosophila . Dev Cell. 2003;5:611–620. doi: 10.1016/S1534-5807(03)00275-2. [DOI] [PubMed] [Google Scholar]
- 136.Furuse M, Tsukita S. Claudins in occluding junctions of humans and flies. Trends Cell Biol. 2006;16:181–188. doi: 10.1016/j.tcb.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 137.Wu VM, Schulte J, Hirschi A, Tepass U, Beitel GJ. Sinuous is a Drosophila claudin required for septate junction organization and epithelial tube size control. J Cell Biol. 2004;164:313–323. doi: 10.1083/jcb.200309134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nelson KS, Furuse M, Beitel GJ. The Drosophila claudin kune-kune is required for septate junction organization and tracheal tube size control. Genetics. 2010;185:831–839. doi: 10.1534/genetics.110.114959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tonning A, Hemphälä J, Tång E, Nannmark U, Samakovlis C, Uv A. A transient luminal chitinous matrix is required to model epithelial tube diameter in the Drosophila trachea. Dev Cell. 2005;9:423–430. doi: 10.1016/j.devcel.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 140.Fehon RG, Dawson IA, Artavanis-Tsakonas S. A Drosophila homologue of membrane-skeleton protein 4.1 is associated with septate junctions and is encoded by the coracle gene. Development. 1994;120:545–557. doi: 10.1242/dev.120.3.545. [DOI] [PubMed] [Google Scholar]
- 141.Hijazi A, Masson W, Auge B, Waltzer L, Haenlin M, Roch F. boudin is required for septate junction organisation in Drosophila and codes for a diffusible protein of the Ly6 superfamily. Development. 2009;136:2199–2209. doi: 10.1242/dev.033845. [DOI] [PubMed] [Google Scholar]
- 142.Llimargas M, Strigini M, Katidou M, Karagogeos D, Casanova J. Lachesin is a component of a septate junction-based mechanism that controls tube size and epithelial integrity in the Drosophila tracheal system. Development. 2004;131:181–190. doi: 10.1242/dev.00917. [DOI] [PubMed] [Google Scholar]
- 143.Wu VM, Yu MH, Paik R, Banerjee S, Liang Z, Paul SM, Bhat MA, Beitel GJ. Drosophila Varicose, a member of a new subgroup of basolateral MAGUKs, is required for septate junctions and tracheal morphogenesis. Development. 2007;134:999–1009. doi: 10.1242/dev.02785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Stucke VM, Timmerman E, Vandekerckhove J, Gevaert K, Hall A. The MAGUK protein MPP7 binds to the polarity protein hDlg1 and facilitates epithelial tight junction formation. Mol Biol Cell. 2007;18:1744–1755. doi: 10.1091/mbc.E06-11-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bachmann A, Timmer M, Sierralta J, Pietrini G, Gundelfinger ED, Knust E, Thomas U. Cell type-specific recruitment of Drosophila Lin-7 to distinct MAGUK-based protein complexes defines novel roles for Sdt and Dlg-S97. J Cell Sci. 2004;117:1899–1909. doi: 10.1242/jcs.01029. [DOI] [PubMed] [Google Scholar]
- 146.Métais J-Y, Navarro C, Santoni M-J, Audebert S, Borg J-P. hScrib interacts with ZO-2 at the cell–cell junctions of epithelial cells. FEBS Lett. 2005;579:3725–3730. doi: 10.1016/j.febslet.2005.05.062. [DOI] [PubMed] [Google Scholar]
- 147.Bilder D, Li M, Perrimon N. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science. 2000;289:113–116. doi: 10.1126/science.289.5476.113. [DOI] [PubMed] [Google Scholar]
- 148.Woods DF, Wu JW, Bryant PJ. Localization of proteins to the apico-lateral junctions of Drosophila epithelia. Dev Genet. 1997;20:111–118. doi: 10.1002/(SICI)1520-6408(1997)20:2<111::AID-DVG4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 149.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ikenouchi J, Furuse M, Furuse K, Sasaki H, Tsukita S, Tsukita S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol. 2005;171:939–945. doi: 10.1083/jcb.200510043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Martìn-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stoppacciaro A, Ruco L, Villa A, Simmons D, Dejana E. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol. 1998;142:117–127. doi: 10.1083/jcb.142.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hirabayashi S, Tajima M, Yao I, Nishimura W, Mori H, Hata Y. JAM4, a junctional cell adhesion molecule interacting with a tight junction protein, MAGI-1. Mol Cell Biol. 2003;23:4267–4282. doi: 10.1128/MCB.23.12.4267-4282.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cohen CJ, Shieh JT, Pickles RJ, Okegawa T, Hsieh JT, Bergelson JM. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc Natl Acad Sci USA. 2001;98:15191–15196. doi: 10.1073/pnas.261452898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nasdala I, Wolburg-Buchholz K, Wolburg H, Kuhn A, Ebnet K, Brachtendorf G, Samulowitz U, Kuster B, Engelhardt B, Vestweber D, Butz S. A transmembrane tight junction protein selectively expressed on endothelial cells and platelets. J Biol Chem. 2002;277:16294–16303. doi: 10.1074/jbc.M111999200. [DOI] [PubMed] [Google Scholar]
- 155.Steed E, Rodrigues NTL, Balda MS, Matter K. Identification of MarvelD3 as a tight junction-associated transmembrane protein of the occludin family. BMC Cell Biol. 2009;10:95. doi: 10.1186/1471-2121-10-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Haskins J, Gu L, Wittchen ES, Hibbard J, Stevenson BR. ZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occludin. J Cell Biol. 1998;141:199–208. doi: 10.1083/jcb.141.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wei X, Ellis HM. Localization of the Drosophila MAGUK protein Polychaetoid is controlled by alternative splicing. Mech Dev. 2001;100:217–231. doi: 10.1016/S0925-4773(00)00550-5. [DOI] [PubMed] [Google Scholar]
- 158.Ide N, Hata Y, Nishioka H, Hirao K, Yao I, Deguchi M, Mizoguchi A, Nishimori H, Tokino T, Nakamura Y, Takai Y. Localization of membrane-associated guanylate kinase (MAGI)-1/BAI-associated protein (BAP) 1 at tight junctions of epithelial cells. Oncogene. 1999;18:7810–7815. doi: 10.1038/sj.onc.1203153. [DOI] [PubMed] [Google Scholar]
- 159.Hamazaki Y, Itoh M, Sasaki H, Furuse M, Tsukita S. Multi-PDZ domain protein 1 (MUPP1) is concentrated at tight junctions through its possible interaction with claudin-1 and junctional adhesion molecule. J Biol Chem. 2002;277:455–461. doi: 10.1074/jbc.M109005200. [DOI] [PubMed] [Google Scholar]
- 160.Lemmers C, Médina E, Delgrossi M-H, Michel D, Arsanto J-P, Le Bivic A. hINADl/PATJ, a homolog of discs lost, interacts with crumbs and localizes to tight junctions in human epithelial cells. J Biol Chem. 2002;277:25408–25415. doi: 10.1074/jbc.M202196200. [DOI] [PubMed] [Google Scholar]
- 161.Citi S, Sabanay H, Jakes R, Geiger B, Kendrick-Jones J. Cingulin, a new peripheral component of tight junctions. Nature. 1988;333:272–276. doi: 10.1038/333272a0. [DOI] [PubMed] [Google Scholar]
- 162.Ohnishi H, Nakahara T, Furuse K, Sasaki H, Tsukita S, Furuse M. JACOP, a novel plaque protein localizing at the apical junctional complex with sequence similarity to cingulin. J Biol Chem. 2004;279:46014–46022. doi: 10.1074/jbc.M402616200. [DOI] [PubMed] [Google Scholar]
- 163.Suzuki A, Ohno S. The PAR-aPKC system: lessons in polarity. J Cell Sci. 2006;119:979–987. doi: 10.1242/jcs.02898. [DOI] [PubMed] [Google Scholar]
- 164.Balda MS, Garrett MD, Matter K. The ZO-1-associated Y-box factor ZONAB regulates epithelial cell proliferation and cell density. J Cell Biol. 2003;160:423–432. doi: 10.1083/jcb.200210020. [DOI] [PMC free article] [PubMed] [Google Scholar]