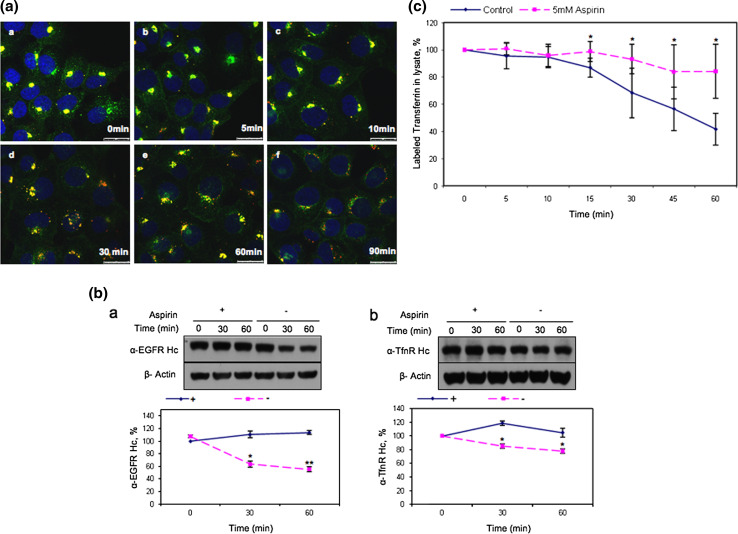
Fig. 4.
Aspirin delays the degradation of EGFR and the recycling of TfnR to the cell surface. a Cells were starved 12 h prior to incubation with Alexa Fluor 555-conjugated EGF and anti-TfnR for 1 h on ice. Thereafter, receptor–antibody complexes were allowed to be endocytosed and accumulated in AMC in the presence of 10 mM aspirin for 4 h. Upon removal of aspirin, cells were washed with PBS and further incubated in media at 37°C, fixed at the indicated time points and stained with secondary antibodies. Images of confocal microscope, scale bars 25 μm. b Cells were incubated with primary antibodies [anti-EGFR, clone 29.1.1 (a) or anti-TfnR(b)] on ice for 1 h, followed by treatment with 10 mM aspirin for 4 h. Subsequently, cells were further incubated with (+) or without (−) 10 mM aspirin and lysed at indicated time points prior to WB analysis. Receptor-specific antibody heavy chain (anti-EGFR Hc or anti-TfnR Hc) were detected with HRP-conjugated anti-mouse IgG. The amount of endocytosed receptors in each sample was normalized to zero time point. Points average of normalized values from three independent experiments; bars ±SD. Results with significant statistical difference from untreated sample: *p < 0.05 and **p < 0.001. c Transferrin recycling assay was performed. The percentage of labeled transferrin in each sample was normalized to time point zero. Points average of normalized values from five independent experiments; bars ±SD. Results with significant statistical difference from untreated sample: *p < 0.05 and **p < 0.001