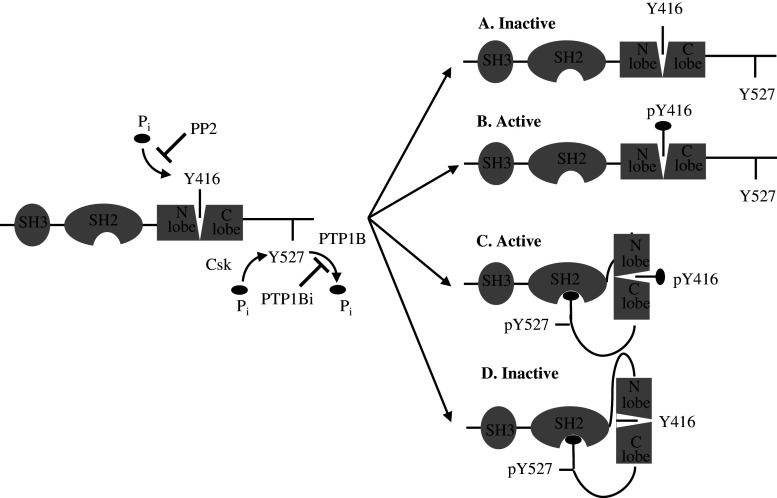
Fig. 6a–d.
Phosphorylation sites and organization of Src kinase family. Src kinases possess an SH3 domain directing binding to specific adaptors or substrates and an SH2 domain containing a phosphotyrosyl-binding pocket and linked to the N lobe. The autocatalytic Y416 residue in C-lobe lies in a pocket between the two lobes. The two lobes can move and open or close the catalytic site. Src can be non-phosphorylated (a), phosphorylated on Y416 residue (b), phosphorylated on both Y416 and Y527 residues (c), and phosphorylated on Y527 residue. Our data showed that a was found in thawed, untreated mitochondria, b and c in ATP-treated mitochondria, and d in PP2- and PTP1Bi-treated mitochondria. ATP induced both autophosphorylation of Y416 residue and Csk-mediated phosphorylation of Y527 residue. PP2 blocks the ATP binding site in the catalytic domain of Src thus leading to inhibition of autophosphorylation without preventing Csk-mediated phosphorylation of Y527. PTP1Bi inhibits PTP1B-mediated dephosphorylation of Y527 residue, leading to interaction of pY527 with the SH2 binding pocket, thus sequestering Y416 residue, which cannot be phosphorylated