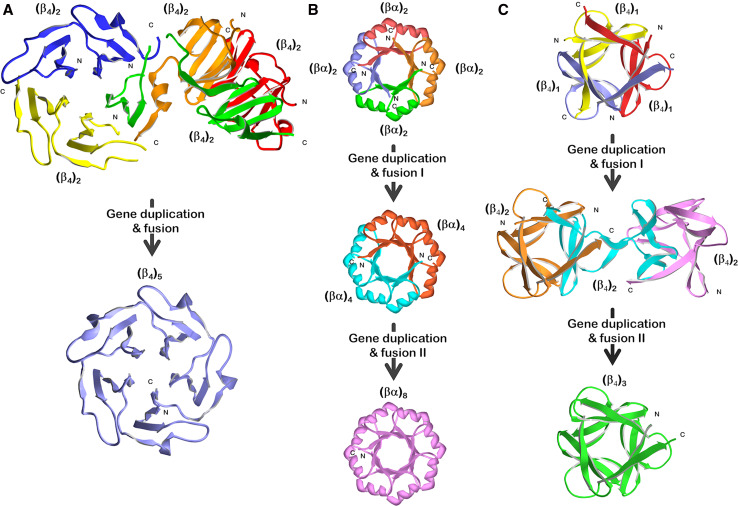
Fig. 2.
Experimental studies of the folding and structural properties of simple peptide motifs derived from three common symmetric protein folds and the relationship to plausible gene duplication and fusion events in their evolution. a Tandem repeats of a ~50-amino-acid (β4) motif from a five-bladed β-propeller fold [21, 26, 30]; b tandem and quadruplicate repeats of a ~35-amino-acid (βα) motif from a (βα)8-barrel fold [28, 35, 38, 59–61]; c single, tandem, and triple repeats of a ~40-amino-acid (β4) motif from a β-trefoil fold [29, 47]. Coloring is used to indicate individual polypeptide chains