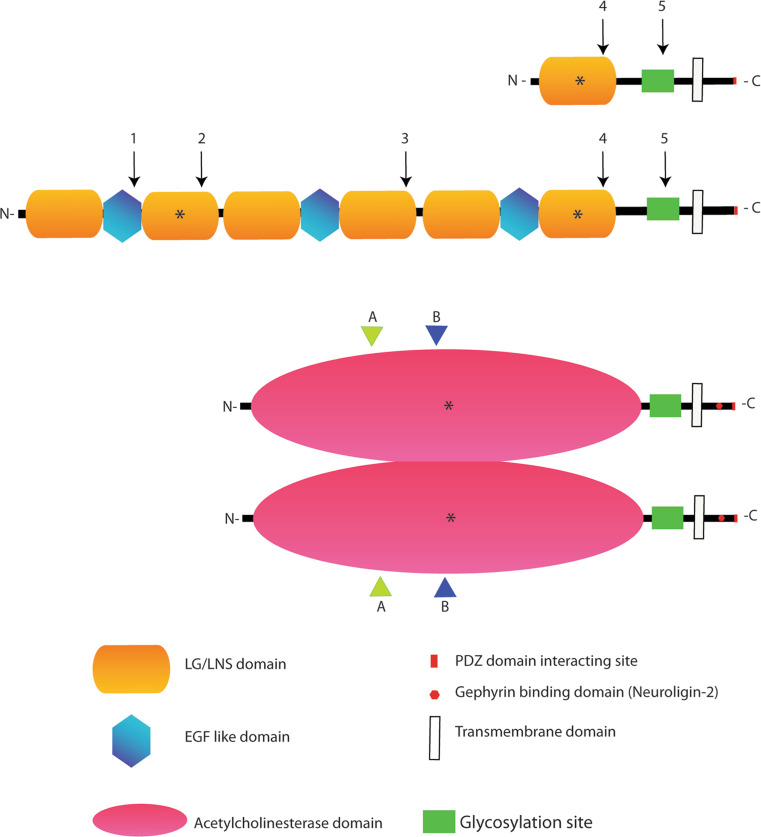
Fig. 1.
Neurexin and Neuroligin protein structures, interactions domains and alternative splicing sites. The biochemical interaction sites for the two proteins are indicated by an asterisk. In the extracellular region of Neurexin, the second LG/LNS domain of the α isoforms binds α-distroglican, while the sixth LG/LNS domain of α-Neurexin and the only LG/LNs domain of β-Neurexin mediate the binding with various Neuroligin isoforms, α- distroglycan and a member of the leucine-rich repeat transmembrane neuronal proteins (LRRTM2). In the case of Neuroligin, the acetylcholinesterase domain is involved in Neuroligin dimerization and binding to all Neurexin isoforms. The intracellular C-terminal region of Neurexin binds to the PDZ domain containing protein CASK while the Neuroligin C-terminal domain binds to the PDZ containing proteins PSD-95 and S-SCAM. Finally, an intracellular stretch of 15 aminoacids in Neuroligin 2 binds Gephyrin. Neuroligin is displayed as a dimer since this is the accepted functional form of the protein [16, 17]. The position of the alternatively spliced sites are indicated by numbers (1–5) in the case of Neurexins and by letters (A–B) in the case of Neuroligins (with splice site B restricted to Neuroligin 1). Splicing variants in the site B of Neuroligin 1 and site splice 4 (SS4) in β Neurexin control the interaction between these proteins. Neurexin 1β SS4+, as Neurexin 1α, has less affinity for Neuroligin 1 with splice site B and binds Neuroligin 2, while Neurexin 1β SS4− binds with high affinity Neuroligin 1 in all the isoforms [10, 19–23]