Fig. 9.
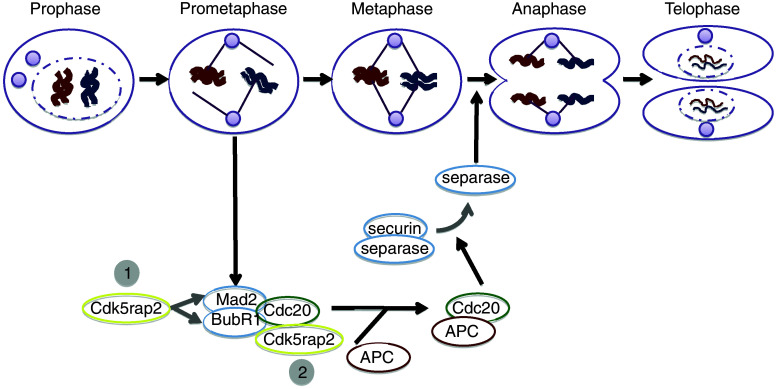
Cdk5rap2 and spindle checkpoint function: a hypothesis. The mitotic spindle checkpoint prevents chromosome missegregation by delaying sister chromatid separation and thus entry of a mitotic cell into anaphase until all chromosomes are bipolarly attached via their kinetochores to spindle microtubules. Chromosome segregation is initiated by spindle checkpoint target APC, which is activated by binding to its activator CDC20 and drives cells from metaphase into anaphase by inducing degradation of securin and mitotic cyclins. The spindle checkpoint proteins BUBR1 and MAD2 are part of an inhibitory complex for APC by sequestering CDC20 through directly binding and thus inhibiting APC activity. It has been suggested that Cdk5rap2 downregulation reduces its binding to CDC20 and inhibits transcription of BUBR1 and MAD2, consequently decreases BUBR1 and MAD2 protein levels, and releases BUBR1-and MAD2-sequestered CDC20. Thereby, more free CDC20 would be available to bind chromatin, to activate the APC and thus to allow transition from metaphase to anaphase in the presence of unattached kinetochores and/or kinetochores that lack tension in CDK5RAP2-inhibited cells