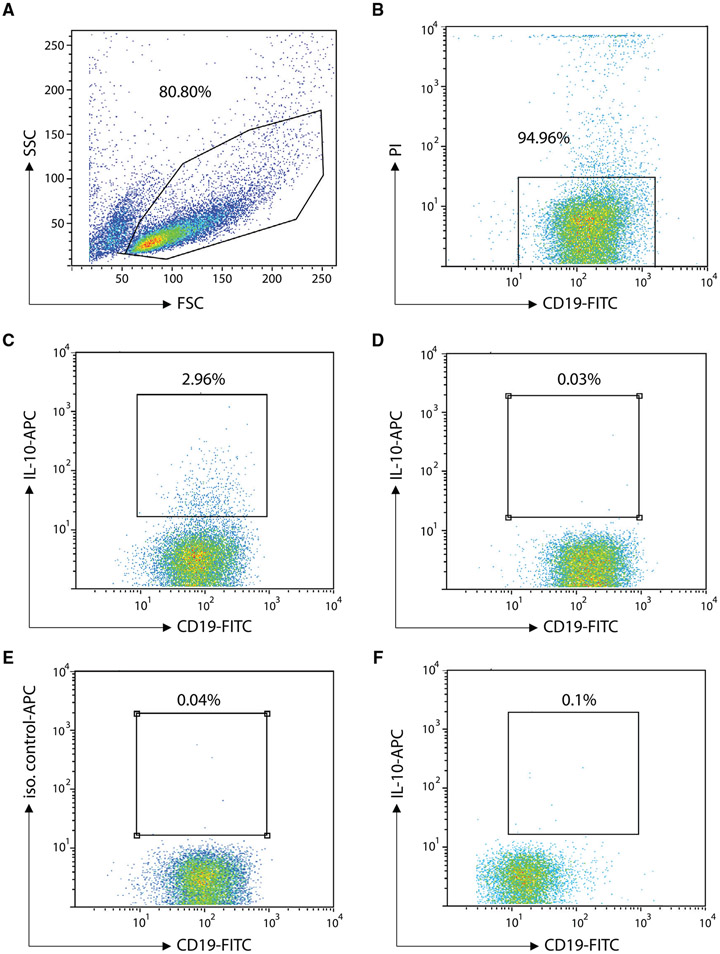
Figure 118.
Detection of IL-10 secreting human B cells. CD19+ B cells were cultured at 2.5x 106/ml in RPMI1640 medium supplemented with 10% FCS and Pen/Strep for 2 days in the presence of 2 μg/ml anti-IgM/IgG-F(ab)2 fragments (Jackson ImmunoResearch), 1 μg/ml anti-CD40 (clone 82111 R&D systems), 10 ng/ml IL-4 (Immunotools, Germany). After a restimulation with 10 ng/ml PMA and 1 μM Ionomycin for 3 hours, the cells were labeled with a bivalent IL-10 capture matrix and the IL-10 secretion period was performed at 37°C for 30 min. After washing, the cells were stained and analyzed with a flow cytometer. Cells were first gated according to FSC/SSC (linear scales) to remove debris and dead cells from the analysis (A). Live CD19+ B cells were then identified within this FSC/SSC gate according to the lack of propidium iodide labeling and CD19 staining (log scales) (B). Dot plots gated on live CD19+ lymphocytes then show CD19 and IL-10 staining (log scales) for stimulated cells with secretion period and IL-10 staining (C). Negative controls include (D) stimulated cells with no secretion period and IL-10 staining, (E) stimulated cells with secretion period and isotype control staining, (F) unstimulated cells with IL-10 staining. Data derived from a representative experiment.