Fig. 2.
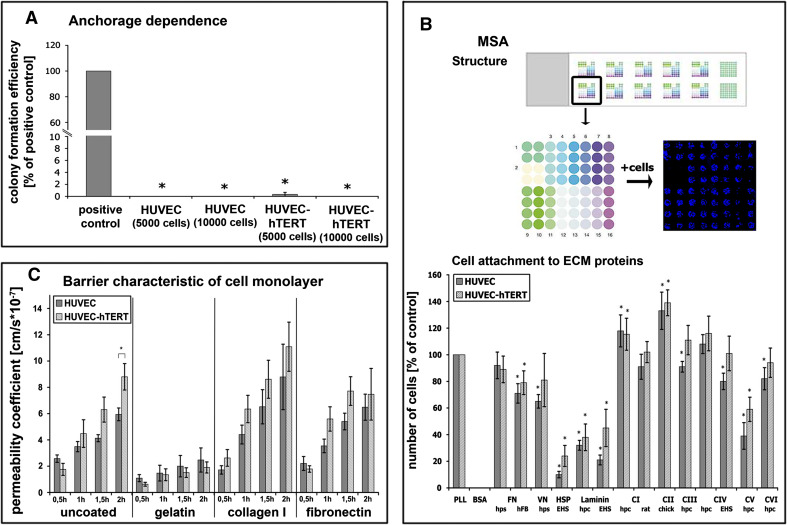
Anchorage dependence: Colony-forming efficiency (a) as an index of malignancy was investigated in soft agar. Only tumor cells without anchorage dependence will grow. N2A cells were used as positive control and displayed a clearly detectable colony-forming potential. In contrast, neither primary nor immortalized HUVECs displayed colony-forming properties. ECM-dependent cell attachment: Experimental layout (b upper panel). Multiple substrate array (MSA) with 12 arrays of 64 microspots each. Each microspot represents one extracellular matrix (ECM) protein; 14 different ECM proteins in quadruplets. 1 PLL, 2 BSA, 3/4 fibronectin (FN), 5 vitronectin (VN), 6 heparan sulfate proteoglycan, 7/8 laminin, 9/10 collagen type I (C I), 11 collagen type II (C II), 12 collagen type III (C III), 13/14 collagen type IV (C IV), 15 collagen type V (C V), 16 collagen type VI (C VI). After cell seeding, incubation, fixation and DAPI staining the blue cell nuclei of attached cells were visualized (far right image, second row). Comparative MSA studies with both HUVECs (b lower panel). Strong adhesion is evident to most collagen types, weak adhesion to HSP and laminin. No adhesion to BSA. Primary and immortalized HUVECs displayed similar attachment characteristics. EHS Basement membrane of Engelbreth-Holm-Swarm murine sarcoma, hpc human placenta, hps human plasma, hFB human fibroblasts. Barrier characteristics of cell monolayers: Permeability measurements of 70 kDa FITC-dextran across monolayers of primary and hTERT-transduced HUVECs grown on different ECM components on transwell filters (c). There are essentially no differences between the two cell types. Cells grown on uncoated, collagen I, or fibronectin-coated filters showed higher permeability coefficients compared to cells cultivated on gelatin, indicating that both HUVEC cell types displayed best barrier integrity when cultivated on gelatin. *P < 0.05 with respect to control or PLL