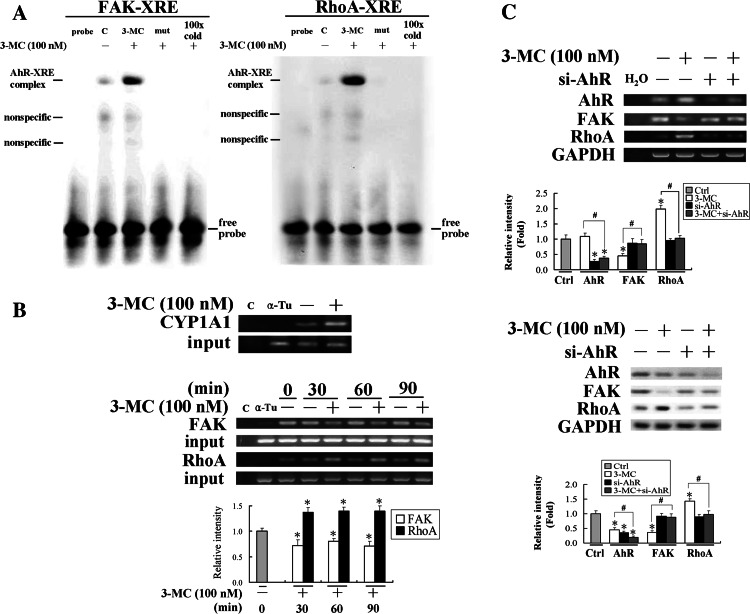
Fig. 6.
The aryl-hydrocarbon receptor (AhR)-dependent mechanism in the alteration of focal adhesion kinase (FAK)/RhoA in human umbilical vascular endothelial cells (HUVECs) challenged with 3-methylcholanthrene (3MC). a EMSA analysis of DNA binding activity of AhR to the putative XREs in FAK/RhoA promoters upon 3MC administration in HUVECs. The putative XREs of FAK/RhoA promoters predicted by MatInspector Professional software and their respective mutants were used as probes in the EMSA assay, as described in “Materials and methods”. Representative results of three separate experiments are shown. b Cells were harvested and subjected to a chromatin immunoprecipitation (ChIP) assay. The DNA associated with the AhR was immunoprecipitated with an anti-AhR antibody, and PCR amplification was used to determine the extent of AhR association with the functional AhR-binding sites (XREs) in the FAK/RhoA promoter fragments of 151 and 162 bp, respectively. CYP1A1 was used as a positive control for the binding of AhR to XRE by 3MC challenge and distilled water (C) and anti-α-tubulin (α-Tu) were respectively used as negative controls for the PCR and ChIP assays. c Cells knocked down with the AhR rescued 3MC-mediated alteration in FAK/RhoA levels. Cells transfected with the siAhR duplex as described in “Materials and methods” were treated with 100 nM 3MC for 1 h, followed by assessment of the expressions of FAK and RhoA at both the mRNA and protein levels. Representative results of three separate experiments are shown and data are presented as the mean ± SD. *Significantly different (P < 0.05 vs the control), #significantly different (P < 0.05 vs 3MC alone)