Abstract
Cells can die by distinct mechanisms with particular impacts on the immune response. In addition to apoptosis and necrosis, recent studies lead to characterization of a new pro-inflammatory form of cell death, pyroptosis. TLR and NLR, central innate immune sensors, can control infections by modulating host cell survival. In addition, TLRs can promote the induction of autophagy, thus promoting delivery of infecting pathogens to the lysosomes. On the other hand, activation of some NLR members, especially NLRC4 and NAIP5, leads to the infected cell death by pyroptosis, which is accompanied by secretion of the pro-inflammatory cytokines IL-1β, IL-18, and IL-33. Data presented here illustrate how the compartmentalization of the innate immune sensors can influence the outcome of infections by controlling the fate of host cells.
Keywords: TLR, NLR, Pyroptosis, Autophagy, Cell death, Inflammasomes
Introduction
Toll-like receptors (TLR) and nucleotide-binding oligomerization domain (NOD), leucine-rich repeats (LRR)-like receptors or NOD-like receptors (NLR), are families of pattern-recognition receptors (PRR) that play a central role in host defense by recognizing pathogen-associated molecular patterns (PAMPs). Several inflammatory genes are activated through TLR and NLR pathways culminating in pathogen control, inflammatory response, and induction of adaptive immune response. Emerging evidence also suggests that the modulation of host cell death by TLR and NLR is another important function of these PRRs. As mediators of the early interaction between pathogens and host cells, TLR and NLR are able to control the fate of host cells by distinct mechanisms. Since different forms of cell death have particular impacts on immune responses, the modulation of cell death processes by PRRs might be considered another important feature of innate immune system. In this review we focused on recent findings regarding the modulation of pyroptosis and autophagy by TLRs and NLRs and, in particular, their consequences for the host resistance to infections.
TLR and NLR: Compartmentalized innate immune sensors
Initial interaction of microorganisms with innate immune cells, especially macrophages (M∅) and dendritic cells (DC), is essential for the induction of the immune response to invading pathogens. The detection of pathogens occurs through the PRRs present on M∅ and DC. Among many families of PRRs, TLRs, and NLRs have been described as central elements in triggering immune responses.
TLR are transmembrane proteins that contain an extracellular leucine-rich repeat (LRR) motifs responsible for ligand recognition and a cytoplasmic Toll/interleukin 1 (IL-1) receptor (TIR) domain responsible for initiating intracellular signaling [1]. TLR engagement by their agonists induces the recruitment of adaptor molecules such as myeloid differentiation factor 88 (MyD88), MyD88-adaptor like/TIR-associated protein (MAL/TIRAP), Toll-receptor-associated activator of interferon (TRIF), Toll-receptor-associated molecule (TRAM), or Sterile α- and armadillo-motif containing protein (SARM) [2]. These adaptor molecules transduce TIR signals, thus activating kinases and transcriptional factors such as nuclear factor (NF)-κB, mitogen-activated protein kinases (MAPKs), and IFN-responsive factors (IRF) [3], culminating in the production of effector molecules, inflammatory cytokines, and type I interferons, responsible for the outcomes of immune responses to PAMPs.
Thirteen TLR family members have been identified in mammals (13 TLR in mice and 11 TLR in humans) [3]. These TLRs sense molecular structures that are distinct from host molecular pattern but frequently found in bacteria, fungi, virus, and some protozoans, such as lipopolysaccharides (LPS), peptideglycans, zymozan, glycosylphosphatidylinositol (GPI), flagellin, profilin, and nucleic acids (double-stranded and single-stranded RNA and unmethylated CpG-DNA).
NLRs have as peculiar characteristic their cytosolic location and comprise several members representing the subfamilies CIITA (MHC II transactivator), NOD, NLRP (NLR family pyrin-domain-containing proteins, also called NALP), NLRC (NLR family CARD-domain-containing), NAIP (neuronal apoptosis inhibitor factors) and NLRX [4]. Similar to TLRs, NLR members contain three domains: an effector and variable N-terminal domain such as the caspase recruitment domain (CARD), the pyrin domain (PYD), the acidic domain, or the baculovirus inhibitor repeats (BIRs); a central NOD-like domain crucial for activation and a C-terminal domain containing leucine-rich repeats, which is assumed to be responsible for the recognition of NLR ligands. Interaction of C-terminal domain with its agonists is thought to induce oligomerization of the central domain bringing in close proximity the effector domain of NLR with adaptor molecules such as RICK (receptor-interacting caspase-like apoptosis-regulatory kinase, also called receptor-interacting protein 2 (Rip2)) or ASC (apoptosis-associated speck-like protein containing a CARD). These cascades culminate in the activation of NF-κB or caspase-1, depending on the type of NLR recruited.
Many aspects of NLR activation remain poorly understood. NOD1 and NOD2, the first described NLR members, are activated in response to muropeptides, products of peptidoglycan degradation [5, 6], inducing a signaling cascade through CARD–CARD interactions between NODs and RICK, which leads to the MAPK or NF-κB activation. Other NLR family members form inflammasomes, oligomeric multi-protein complexes composed of a member of NLR family, and adaptor molecule such as ASC [7]. Assembly of inflammasomes leads to the recruitment and activation of pro-caspase-1 resulting in caspase-1-dependent IL-1β, IL-18, IL-33 secretion and pyroptosis induction [8, 9].
Four NLR family members have been described as components of inflammasomes: NLRP1b (also called NALP1), NLRP3 (also known as NALP3), NLRC4 (or IPAF), and NAIP5. To date, the best-characterized inflammasome complex is NLRP3. Activation of this NLR leads to the recruitment of the downstream components, ASC and caspase-1. Inflammasome assembly occurs through the interaction between the NLRP3 PYD domain and the PYD domain present in ASC carboxy-terminal domain that, in turn, activates pro-caspase-1 through its amino-terminal CARD domain [8].
NLRP3 inflammasomes are activated in response to many stimuli, such as ATP and bacterial pore-forming toxins [10], urate crystals [11] asbestos and silica [12], bacterial muramyl dipeptide (MDP) [13, 14], LPS [10, 15, 16], bacterial DNA and RNA [17, 18], viral RNA and imidazoquinolone antiviral compounds R837 and R848 [17, 19], zymosan and mannan, components of fungal cell wall [20] and hemozoin from the protozoan Plasmodium [21, 22]. The mechanism by which NLRP3 detects this diversity of signals is unknown since they are not structurally related [9]. NLRP3 activation in vitro generally requires two signals: one is provided by a TLR ligand, and the other by the many stimuli that can cause loss of plasma membrane or lysosomal membrane integrity. The loss of membrane integrity appears to be sensed through potassium ion efflux. Induction of potassium efflux by ATP acting through its receptor P2X7, can also cause NLRP3 inflammasome activation.
NLRP1b inflammasome assembly in response to anthrax lethal toxin (LT) was recently described to comprise NLRP1, caspase-1, caspase-11, α-enolase but not ASC [23]. Surprisingly, LT seem to trigger NLRP1b inflammasome formation at the cell membrane, which induces caspase-1 activation and macrophage lysis. Similar to NLRP1, NLRC4 can also directly interact and activate caspase-1 via its amino-terminal CARD domain [8, 24]. However, ASC participation can not be completely discarded since its absence reduces NLRC4-mediated caspase-1 activation in response to Salmonella infection [25].
Interactions mediated by NAIP5 protein in the assembly of inflammasome remain to be determined. NAIP5-deficient macrophages have defective caspase-1 activation [26] but instead of CARD or PYD amino-terminal domain, NAIP5 possesses a BIR domain. One possible explanation for NAIP5-mediated caspase-1 activation is through its interaction with NLRC4 [27]. However, NAIP5 does not share with NLRC4 all caspase-1-mediated functions [26]. Thus, the possibility of interaction between NAIP5 and another adaptor molecule can not be discarded. Furthermore, the possibility of multiple interactions observed among NLR members and adaptor molecules supports the idea that multiple complexes containing more than one NLR member can be found within the same cell [28]. However, the influence of cross-talk between different NLR for host cell survival and resistance to infections remains to be determined.
In contrast to a broad range of diverse stimuli that can activate the NLRP3 inflammasome, NLRC4 and NAIP5 inflammasomes are activated mainly by cytosolic flagellin, a monomeric subunit of flagella present in most Gram-negative and Gram-positive bacteria. The delivery of flagellin to cell cytosol requires a functional secretion system such as T3SS (type III secretion system) for S. typhimurium [29, 30], S. flexneri [31], and P. aeruginosa [32, 33], and T4SS (type IV secretion system) for L. pneumophila [27, 34]. The intracellular bacterium Listeria monocitogenes has also been demonstrated to activate NLRC4 through flagellin sensing [35]. Both NLRC4 and NAIP5 recognize the same 35 amino acids of a peptide located in the C-terminal D0 domains of L. pneumophila and S. typhimurium flagellins, distinct from the D1 domain sensed by transmembrane TLR5 [26]. However, NLRC4 inflammasome is also activated by certain bacteria such as S. flexneri [31] and P. aeruginosa [33], independently of flagellin, via still unknown agonists.
Thus, the recognition of PAMPs by transmembrane TLR induces activation of anti-microbial innate immune responses, inflammation, and adaptive immune responses [4, 36]. Activation of NOD1 and NOD2 can result in similar outcomes. On the other hand, NLRP1, NLRP3, NLRC4, and NAIP5 proteins initiate inflammatory response through caspase-1 activation, secretion of IL-1β, IL-18 and IL-33 and induction of inflammatory form of programmed cell death known as pyroptosis [9] (Fig. 1).
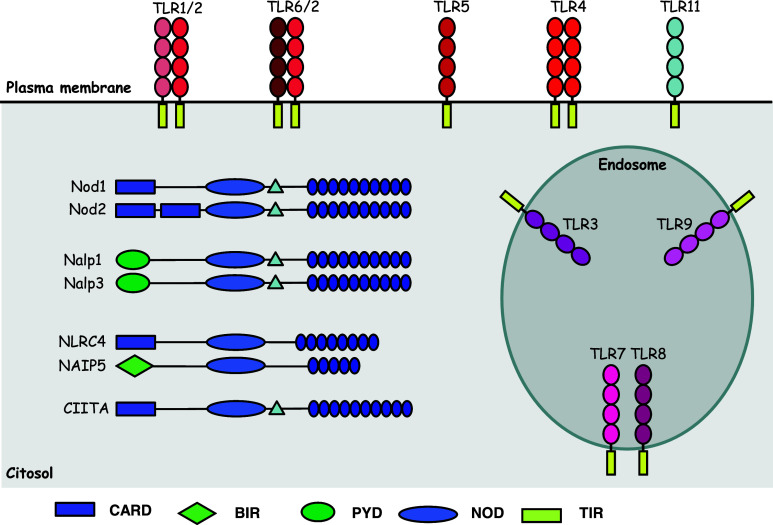
Fig. 1.
Compartmentalized innate immune sensors. TLR are transmembrane proteins that can be found at plasma membrane (TLR1, 2, 4, 5, 6, and 11) or at endosomes (TLR3, 7, 8, and TLR9). These proteins contain an extracellular domain containing leucine-rich repeat (LRR) motifs responsible for ligand recognition and a cytoplasmic Toll/interleukin-1 (IL-1) receptor (TIR) domain that recruits adaptor molecules (not represented here) thereby initiating intracellular signaling [1]. NLRs are cytosolic proteins that contain three domains: a C-terminal domain containing LRR, which is assumed to be responsible for the recognition of NLR ligands; a central NOD-like domain crucial for activation; and a diverse effector N-terminal domain, such as the caspase recruitment domain (CARD), the pyrin domain (PYD), the acidic domain, or the baculovirus inhibitor repeat (BIR) domain
Different forms of cell death and their influence on the outcome of immune responses
Besides the well-known distinctions between apoptosis and necrosis, recent studies have uncovered additional cell-death pathways with distinct roles in inflammation and host defense. Apoptosis is the best characterized form of cell death, which is a programmed process regulated at molecular levels by cysteinyl aspartate-specific proteases called caspases (discussed in more detail in Schrader et. al., in this issue). Morphological features characteristic in apoptosis include DNA and nuclear fragmentation, loss of cell volume, formation of cytoplasmic and membrane blebs, packaging of cellular contents and phosphatidylserine externalization. More importantly, apoptotic cells are rapidly removed by neighboring phagocytes without causing inflammatory response.
In contrast to apoptosis, overwhelming stress induces an uncontrolled process of cell death resulting in morphological changes capable of causing strong inflammation. This form of cell death used to be known as necrosis (for more information we refer to Van Herreweghe et. al., in this issue). Necrotic cells increase their volume and permeability, maintain the uncondensed DNA content, and lose their cellular contents including uric acid, adenine triphosphate, purine metabolites, high-mobility group box 1 protein (HMGB-1), heat shock proteins (HSP) among others, which activate immune cells. However, the improvement in available technologies to evaluate cell death and the current understanding of this field permit to draw another scenario in which a caspase-independent form of cell death with morphological features from necrosis has been described as a molecularly regulated process. RIP1, a death-domain-containing kinase associated with the death receptors seems to be dispensable for the induction of death-receptor-mediated apoptosis [37], but it is essential for this caspase-independent cell death, suggesting the concept of a distinct mechanism of regulated cell death from apoptosis [38, 39].
Among these polarized processes of cell death, other pathways retaining morphological features from both apoptosis and necrosis have been described. Pyroptosis is a programmed cell death that uniquely depends on the inflammatory caspase-1 activity [28]. Caspase-1 is not involved in apoptosis but it is essential for maturation and secretion of pro-inflammatory cytokines IL-1β, IL-18, and IL-33 [8]. On the other hand, inhibition or deficiency in effector caspases involved in apoptosis does not rescue cells from pyroptosis. Like apoptotic caspases, caspase-1 is synthesized as an inactive pro-caspase and its cleavage and activation occurs within the inflammasome oligomeric structure [9]. Since inflammasome complexes comprise a member from the NLR family that functions as a starter, pyroptosis has been considered as an infection-induced cell death. Besides being a caspase-regulated process like apoptosis, pyroptosis has distinct outcomes for immune response that might be explained by the morphological changes induced by this pathway. Pyroptotic cells undergo DNA fragmentation and nuclear condensation like apoptotic cells but the most important feature of this process is the secretion of inflammatory mediators, including IL-1β, IL-18, and IL-33, which are responsible for the inflammatory response that accompanies this form of cell death.
Autophagy is another programmed process that might culminate in cell death. In fact, autophagy is mainly a protective process induced under stress conditions by which cells engulf large portions of their own cytoplasm or damaged organelles [40]. This pathway is essential for nutrient recycling and toxic metabolite degradation, and therefore important in maintaining cell viability under starvation and stress conditions. However, when excessive, autophagy culminates in cell death. Threonine serine kinase mTOR is the molecular target responsible for the regulation of autophagy machinery. In the absence of nutrients or other cellular stress conditions, mTOR is inhibited, inducing the process of autophagosome formation orchestrated by the ATG/Beclin proteins. VPS34 [a class III phosphoinositide 3-kinase (PI3K)]/Beclin complex initiates phagophore formation, a structure composed by double membrane. Then, the Atg5/12/16 complex is recruited to phagophore providing a docking site for LC3 (light chain 3)-II, the product of the second ubiquitin-like system. The recruitment of LC3-II elongates the phagophores, inducing the appearance of a double membrane organelle called an autophagosome, which fuses with lysosome originating autolysosome that degrades ingested cellular contents. Intense vacuolization is the major morphological feature of autophagic cells that do not lose membrane integrity. Therefore, contrary to pyroptosis and necrosis, autophagy is a silent cell death process.
In this scenario, each of these cell-death pathways possesses its own molecular machinery responsible for a particular influence on the immune response. Emerging evidence suggests that the influence of TLRs and NLRs in all of these pathways might occur during infections, in an independent fashion, or concomitantly. Since the result of cell death is not always positive for the host, an understanding of the molecular mechanisms responsible for determining the type of cell death after TLR and NLR engagement is one of the major challenges in this field.
Influence of inflammasomes on infection control through cell death
Inflammasomes, platforms that culminate in caspase-1 activation, are central in pyroptosis induction. Conversely, TLRs seem not to be directly involved in the induction of pyroptosis, since the known signaling pathways activated by these proteins do not culminate in caspase-1 activation. However, priming of macrophages with TLR agonists, such as LPS and Pam3CSK, can redirect cell death from apoptosis towards pyroptosis during Yersinia pseudotuberculosis infection [41]. Since cell death by pyroptosis is followed by pro-inflammatory cytokine secretion and cellular contents release, this interaction between TLR and NLR is an additional illustration of their synergism in order to improve host resistance to infections. This inflammatory process of cell death warrants the recruitment of effector cells to the site of infection, thus contributing to pathogen clearance. Moreover, by inducing pyroptosis during an infection, the host could limit microbial dissemination through the elimination of their niche. However, the idea of pyroptotic cell death as an effector mechanism of the host cell to control infections by itself is a matter of debate [9, 28, 42].
By inducing caspase-1 activation, all known inflammasomes can induce pyroptosis, even though the consequences for the host cell might be distinct in each case. NLRP1b was reported to recognize anthrax lethal toxin leading to caspase-1-mediated macrophage cell death [23, 43]. However, the consequence of this cell death is controversial. Despite the fact that the pyroptosis mimics the toxic effect of anthrax lethal toxin, an important function for the NLRP1b inflammasome in host defense is suggested by the increased susceptibility to infection with B. anthracis observed in mice with the Nlrp1b susceptibility allele [44].
Listeria monocitocitogenes, Staphilococus aureus [10], Saccharomyces cerevisae [20], Candida albicans [45, 46], Influenza and Sendai viruses [18] have all been reported to activate caspase-1 through NLPR3, suggesting an important role for the NLRP3 inflammasome during bacterial, viral, and fungal infections. However, there is no direct evidence of NLRP3-mediated pyroptosis in the control of these pathogens. Moreover, during Plasmodium chabaudi adami [22] and Plamodium berghei ANKA [21] infections, instead of parasite control, inflammatory response induced by NLRP3 is implicated in the development of pathology such as fever, body weight loss, cerebral malaria, and mortality.
NLRC4-mediated caspase-1 activation has also been linked to the induction of inflammation and pyroptosis. In this case, pyroptosis seems to be involved in the macrophage clearance of some bacterial pathogens such as Salmonella typhymurium [29, 30], Pseudomonas aeruginosa [47], and Shigella flexneri [31], since the absence of NLRC4 or caspase-1 renders macrophages resistant to cell death and susceptible to infections. An effector role of pyroptosis was also demonstrated to NAIP5-dependent control of Legionella pneumophila [27] in which macrophages from NAIP5 mutant mice (A/J) exhibited an impaired caspase-1 activation followed by defective pyroptosis and microbial control.
Besides this evidence, it is important to make it clear that the direct contribution of pyroptosis to macrophage microbicidal capacity is still restricted only to a few bacterial infections and its relevance in vivo is unknown at present. Furthermore, the activation of NAIP5/NLRC4-caspase-1 axis by flagellin induces other effector mechanisms to restrict infections, such as phagosome maturation leading to the inhibition of L. pneumophila replication [34, 48] and the activation of inducible nitric oxide synthase (iNOS) by bone marrow-derived (BMDM) and peritoneal macrophages (Buzzo, CL and Bortoluci, KR; unpublished data). NLRC4/NAIP5-caspase-1 seems to mediate the maturation of L. pneumophila-containing phagosome through caspase-7 activation [48]. BMDM from casp7−/− mice are permissive to L. pneumophila replication, which seems to be correlated with the reduced rate of lysosomal-associated membrane protein-1 (LAMP-1) present in their phagosomes. Importantly, caspase-1-induced cytokines, IL-1β and IL-18, are not involved in this feature, since IL-1−/−/IL-18−/− BMDM are able to induce caspase-7 activation in response to L. pneumophila. Similarly, iNOS activation induced by cytosolic flagellin is not inhibited in the presence of neutralizing antibodies to IL-1β or IL-1R (Buzzo, CL and Bortoluci, KR; unpublished data). Taken together, these data suggest that the features mediated by caspase-1, such as pyroptosis, pro-inflammatory cytokines secretion, caspase-7, and iNOS activation could occur as independent events that might assist with the clearance of pathogens.
Autophagy in the host control of infections
Autophagy is a well-characterized process that protects host cells from nutrient deprivation and other cellular stresses. However, increasing evidence indicates the potential of autophagy in controlling infections by directing intracellular or ingested pathogens to lysosomes leading to their destruction [49]. Notably in this regard, stimulation of macrophages with soluble TLR agonists such as Pam3CSK4 (TLR1), Pam2CSK4 (TLR2), poly(I:C) (TLR3), LPS (TLR4), flagellin (TLR5), MALP-2 (TLR6), ssRNA (TLR7) and CpG oligodeoxy nucleotides (TLR9) was capable to enhance LC3II puncta inside macrophages, increase LC3-I to LC3-II conversion and the presence of double membrane-phagosomes, characteristics of conventional autophagy [50, 51].
Despite the fact of all TLR ligands seem to have an intrinsic capacity to induce autophagy, they do not all have the same effect. Among them, TLR7 ligands are the most potent inducers of autophagy, and the reason for its particular effect is still not clear [50]. Induction of autophagy by TLRs involves the activation of both MyD88 and TRIF-mediated pathways by a mechanism that has been partially elucidated. TLR stimulation induces the interaction of Beclin-1, a key inducer of autophagosome formation, with MYD88 and TRIF adaptor molecules. This interaction, in turn, dissociates Beclin-1 from anti-apoptotic proteins Bcl-2 and Bcl-XL, thus leading to the induction of autophagy [51].
In addition to the participation of TLR signaling in the conventional autophagy, these proteins seem to mediate another unconventional process involving autophagy machinery named LC3-assisted phagocytosis (LAP) [52]. In LAP, the ingestion of cargos containing TLR ligands such as zymozan particles or TLR agonists-coated beads, but not inert ones, induces the rapid recruitment of LC3 to the surface of the ingested phagosome. Importantly, these LC3-positive phagosomes do not present the double membrane that characterizes autophagosomes. Similar to TLR-induced conventional autophagy, TLR-induced LAP also results in an accelerated maturation of cargo-containing phagosomes, therefore reducing opportunities for microorganisms to subvert the phagosome and improving macrophage control of infections [49].
The role of NLR in the induction of autophagy is not as clear as TLR. It has been demonstrated that during Shigella infection, macrophages can undergo autophagy [31]. Interestingly, the induction of autophagy by Shigella requires the same NLR member NLRC4 implicated in pyroptosis. However, in this case, there is a regulatory cross-talk between these two processes in which caspase-1 activation inhibits autophagy. Moreover, LPS stimulation of macrophages deficient in Atg16L1 (autophagy-related16-like 1), a critical component of autophagic machinery, induces an enhanced TRIF-dependent activation of caspase-1 resulting in the secretion of high levels of IL-1β [53]. Since the enhanced production of IL-1β noted in Atg16L1-deficient macrophages requires both potassium efflux and the generation of reactive oxygen species, which activate NLRP3, autophagy also could negatively regulate inflammasomes.
Despite the little data clarifying the pathways coordinating TLR and NLR with autophagy, emerging evidence supports the role of this process in host defense. Pathogens capable of inhibiting the fusion of the phagosome to the lysosome such as Mycobacterium tuberculosis and Toxoplasma gondii have been shown to be controlled by autophagy [54–56]. Even for infections that efficiently induce pyroptosis, the autophagic pathway seems to participate. This is the case for Salmonella [57, 58], Legionella [59], Listeria [60], and Francisella [61]. However, many pieces of this complex network of effector mechanisms against infections induced by PRR, especially TLR and NLR, are still missing. A great challenge in this scenario is to understand how the host–pathogen interaction works to permit the predominant activation of a particular effector mechanism.
Concluding remarks
The process by which host cells die has a specific impact on the immune response. In addition to relatively well-characterized apoptosis and necrosis, other forms of cell death have recently gained importance. Moreover, the families of PRR, TLR, and NLR seem to orchestrate many aspects of innate immunity by inducing host cell death. Taken together, the information discussed here suggests to the following key points:
Autophagy and pyroptosis could be included in another polarized scheme similar than apoptosis versus necrosis, since they have opposite consequences to the immune response. Moreover, this new scheme could be inserted in the middle of the polarized apoptosis versus necrosis, since they share some molecular characteristic with both processes (Fig. 2).
The influence of TLRs on the induction of autophagy is evident. On the other hand, cell death by pyroptosis is orchestrated by inflammasomes, which seem to negatively modulate autophagy. Since NLR are cytosolic sensors, many pathogens must possess virulent factors that disrupt plasma membranes or phagosomes to reach cell cytoplasm where they are detected by these proteins. Then, we can speculate that inflammasomes are the sensors mainly for pathogenic microorganisms. Consequently, the induction of pyroptosis, a high inflammatory process of cell death might be considered an aggressive response of the innate immune system for these pathogens. Maybe under less dangerous situations the activation of the non-inflammatory autophagic machinery might lead to a positive result for the host by controlling the microorganism without cost of the cell loss.
Despite the evidence pointing to the contribution of pyroptosis and autophagy to pathogen clearance, there are still many missing pieces. Do these processes have a role during in vivo infections? Is there an interaction between these forms of cell death and between them and other effector mechanisms such as ROIs and RNIs generation, etc? What are the factors that permit the prevalence of a particular effector mechanism against infections? The answers for these questions might not be so simple and easy but they bring more enthusiasm to this fascinating innate immune universe.
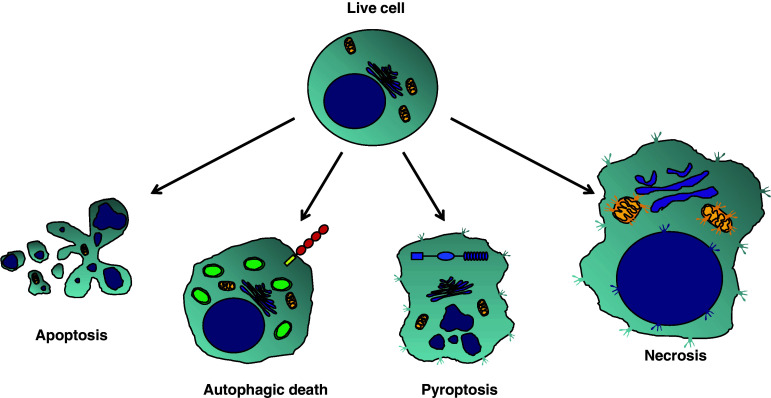
Fig. 2.
Proposed scheme for different forms of cell death. Apoptosis and necrosis apparently are the most distinct forms of cells death. Apoptosis is a strictly regulated process of cell death in which cells undergo some modifications that permits their silent removal by adjacent cells. These modifications include reduction of cell volume and packaging of cellular contents, DNA and nuclear fragmentation, formation of cytoplasmic and membrane blebs, and phosphatidylserine externalization. In contrast, necrotic cells increase their volume and lose the cell membrane integrity leading to the release of their cellular contents and consequent inflammation. Note that necrotic cells preserve the uncondensed DNA content. Autophagy and pyroptosis could be seen as another polarized scheme of cell death, since they have opposite consequences to the immune response. Like necrosis, pyroptosis is a highly inflammatory process in which cells also lose their cell membrane integrity. However, along with cellular contents, pyroptotic cells secrete inflammatory cytokines such as IL-1β, IL-18, and IL-33. Interestingly, the nuclear modifications observed during pyroptosis are similar to the DNA fragmentation and nuclear condensation detected in apoptosis. Similarly to apoptosis, autophagy is another silent process by which cells can undergo death. In this case, cells maintain the membrane integrity and exhibit a profound vacuolization as a result of the double-membrane composed autophagosome formation. Since autophagy and pyroptosis share some molecular characteristics with both apoptosis and necrosis, we inserted this new scheme in the middle of them. Moreover, as TLR clearly modulate autophagy and inflammasomes are the key inducers of pyroptosis, these two families of PRR are represented in cells undergoing their respective forms of cell death
Acknowledgments
KRB is supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Brazil and from the Brazilian Research Council (CNPq). RM is an Investigator at the Howard Hughes Medical Institute.
References
- 1.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 2.Carty M, Goodbody R, Schroder M, Stack J, Moynagh PN, Bowie AG. The human adaptor SARM negatively regulates adaptor protein TRIF-dependent Toll-like receptor signaling. Nat Immunol. 2006;7:1074–1081. doi: 10.1038/ni1382. [DOI] [PubMed] [Google Scholar]
- 3.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Franchi L, Warner N, Viani K, Nunez G. Function of Nod-like receptors in microbial recognition and host defense. Immunol Rev. 2009;227:106–128. doi: 10.1111/j.1600-065X.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inohara N, Koseki T, del Peso L, Hu Y, Yee C, Chen S, Carrio R, Merino J, Liu D, Ni J, Núñez G. Nod1, an Apaf-1-like activator of caspase-9 and nuclear factor-kappaB. J Biol Chem. 1999;274:14560–14567. doi: 10.1074/jbc.274.21.14560. [DOI] [PubMed] [Google Scholar]
- 6.Ogura Y, Inohara N, Benito A, Chen FF, Yamaoka S, Nunez G. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J Biol Chem. 2001;276:4812–4818. doi: 10.1074/jbc.M008072200. [DOI] [PubMed] [Google Scholar]
- 7.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/S1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 8.Martinon F, Tschopp J. Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ. 2007;14:10–22. doi: 10.1038/sj.cdd.4402038. [DOI] [PubMed] [Google Scholar]
- 9.Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10:241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mariathasan S, Weiss D, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee W, Weinrauch Y, Monack D, Dixit V. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 11.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 12.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinon F, Agostini L, Meylan E, Tschopp J. Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome. Curr Biol. 2004;14:1929–1934. doi: 10.1016/j.cub.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 14.Marina-Garcia N, Franchi L, Kim YG, Miller D, McDonald C, Boons GJ, Nunez G. Pannexin-1-mediated intracellular delivery of muramyl dipeptide induces caspase-1 activation via cryopyrin/NLRP3 independently of Nod2. J Immunol. 2008;180:4050–4057. doi: 10.4049/jimmunol.180.6.4050. [DOI] [PubMed] [Google Scholar]
- 15.Kanneganti TD, Lamkanfi M, Nunez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27:549–559. doi: 10.1016/j.immuni.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant EP, Bertin J, Coyle AJ, Galán JE, Askenase PW, Flavell RA. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Muruve DA, Petrilli V, Zaiss AK, White LR, Clark SA, Ross PJ, Parks RJ, Tschopp J. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452:103–107. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]
- 18.Kanneganti TD, Ozören N, Body-Malapel M, Amer A, Park JH, Franchi L, Whitfield J, Barchet W, Colonna M, Vandenabeele P, Bertin J, Coyle A, Grant EP, Akira S, Núñez G. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 19.Kanneganti TD, Body-Malapel M, Amer A, Park JH, Whitfield J, Franchi L, Taraporewala ZF, Miller D, Patton JT, Inohara N, Núñez G. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J Biol Chem. 2006;281:36560–36568. doi: 10.1074/jbc.M607594200. [DOI] [PubMed] [Google Scholar]
- 20.Lamkanfi M, Malireddi RK, Kanneganti TD. Fungal zymosan and mannan activate the cryopyrin inflammasome. J Biol Chem. 2009;284:20574–20581. doi: 10.1074/jbc.M109.023689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dostert C, Guarda G, Romero JF, Menu P, Gross O, Tardivel A, Suva ML, Stehle JC, Kopf M, Stamenkovic I, Corradin G, Tschopp J. Malarial hemozoin is a Nalp3 inflammasome activating danger signal. PLoS One. 2009;4:e6510. doi: 10.1371/journal.pone.0006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shio MT, Eisenbarth SC, Savaria M, Vinet AF, Bellemare MJ, Harder KW, Sutterwala FS, Bohle DS, Descoteaux A, Flavell RA, Olivier M. Malarial hemozoin activates the NLRP3 inflammasome through Lyn and Syk kinases. PLoS Pathog. 2009;5:e1000559. doi: 10.1371/journal.ppat.1000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nour AM, Yeung YG, Santambrogio L, Boyden ED, Stanley ER, Brojatsch J. Anthrax lethal toxin triggers the formation of a membrane-associated inflammasome complex in murine macrophages. Infect Immun. 2009;77:1262–1271. doi: 10.1128/IAI.01032-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poyet JL, Srinivasula SM, Tnani M, Razmara M, Fernandes-Alnemri T, Alnemri ES. Identification of Ipaf, a human caspase-1-activating protein related to Apaf-1. J Biol Chem. 2001;276:28309–28313. doi: 10.1074/jbc.C100250200. [DOI] [PubMed] [Google Scholar]
- 25.Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girmam M, Erickson S, Dixit VM. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 26.Lightfield KL, Persson J, Brubaker SW, Witte CE, von Moltke J, Dunipace EA, Henry T, Sun YH, Cado D, Dietrich WF, Monack DM, Tsolis RM, Vance RE. Critical function for Naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nat Immunol. 2008;9:1171–1178. doi: 10.1038/ni.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zamboni DS, Kobayashi KS, Kohlsdorf T, Ogura Y, Long EM, Vance RE, Kuida K, Mariathasan S, Dixit VM, Flavell RA, Dietrich WF, Roy CR. The Birc1e cytosolic pattern-recognition receptor contributes to the detection and control of Legionella pneumophila infection. Nat Immunol. 2006;7:318–325. doi: 10.1038/ni1305. [DOI] [PubMed] [Google Scholar]
- 28.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, Miller SI, Aderem A. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol. 2006;7:569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 30.Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Ozören N, Jagirdar R, Inohara N, Vandenabeele P, Bertin J, Coyle A, Grant EP, Núñez G. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in Salmonella-infected macrophages. Nat Immunol. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki T, Franchi L, Toma C, Ashida H, Ogawa M, Yoshikawa Y, Mimuro H, Inohara N, Sasakawa C, Nuñez G. Differential regulation of caspase-1 activation, pyroptosis, and autophagy via Ipaf and ASC in Shigella-infected macrophages. PLoS Pathog. 2007;3:e111. doi: 10.1371/journal.ppat.0030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franchi L, Stoolman J, Kanneganti TD, Verma A, Ramphal R, Nunez G. Critical role for Ipaf in Pseudomonas aeruginosa-induced caspase-1 activation. Eur J Immunol. 2007;37:3030–3039. doi: 10.1002/eji.200737532. [DOI] [PubMed] [Google Scholar]
- 33.Sutterwala FS, Mijares LA, Li L, Ogura Y, Kazmierczak BI, Flavell RA. Immune recognition of Pseudomonas aeruginosa-mediated by the IPAF/NLRC4 inflammasome. J Exp Med. 2007;204:3235–3245. doi: 10.1084/jem.20071239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amer A, Franchi L, Kanneganti TD, Body-Malapel M, Ozören N, Brady G, Meshinchi S, Jagirdar R, Gewirtz A, Akira S, Núñez G. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J Biol Chem. 2006;281:35217–35223. doi: 10.1074/jbc.M604933200. [DOI] [PubMed] [Google Scholar]
- 35.Warren SE, Mao DP, Rodriguez AE, Miao EA, Aderem A. Multiple Nod-like receptors activate caspase 1 during Listeria monocytogenes infection. J Immunol. 2008;180:7558–7564. doi: 10.4049/jimmunol.180.11.7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palm NW, Medzhitov R. Pattern recognition receptors and control of adaptive immunity. Immunol Rev. 2009;227:221–233. doi: 10.1111/j.1600-065X.2008.00731.x. [DOI] [PubMed] [Google Scholar]
- 37.Grimm S, Stanger BZ, Leder P. RIP and FADD: two “death domain”-containing proteins can induce apoptosis by convergent, but dissociable, pathways. Proc Natl Acad Sci USA. 1996;93:10923–10927. doi: 10.1073/pnas.93.20.10923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B, Tschopp J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 39.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 40.Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol. 2008;9:1004–1010. doi: 10.1038/nrm2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bergsbaken T, Cookson BT. Macrophage activation redirects yersinia-infected host cell death from apoptosis to caspase-1-dependent pyroptosis. PLoS Pathog. 2007;3:e161. doi: 10.1371/journal.ppat.0030161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ting JP, Willingham SB, Bergstralh DT. NLRs at the intersection of cell death and immunity. Nat Rev Immunol. 2008;8:372–379. doi: 10.1038/nri2296. [DOI] [PubMed] [Google Scholar]
- 43.Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet. 2006;38:240–244. doi: 10.1038/ng1724. [DOI] [PubMed] [Google Scholar]
- 44.Kang TJ, Basu S, Zhang L, Thomas KE, Vogel SN, Baillie L, Cross AS. Bacillus anthracis spores and lethal toxin induce IL-1beta via functionally distinct signaling pathways. Eur J Immunol. 2008;38:1574–1584. doi: 10.1002/eji.200838141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gross O, Poeck H, Bscheider M, Dostert C, Hannesschläger N, Endres S, Hartmann G, Tardivel A, Schweighoffer E, Tybulewicz V, Mocsai A, Tschopp J, Ruland J. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459:433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- 46.Joly S, Ma N, Sadler JJ, Soll DR, Cassel SL, Sutterwala FS. Cutting edge: Candida albicans hyphae formation triggers activation of the Nlrp3 inflammasome. J Immunol. 2009;183:3578–3581. doi: 10.4049/jimmunol.0901323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Franchi L, Kanneganti TD, Dubyak GR, Nunez G. Differential requirement of P2X7 receptor and intracellular K+ for caspase-1 activation induced by intracellular and extracellular bacteria. J Biol Chem. 2007;282:18810–18818. doi: 10.1074/jbc.M610762200. [DOI] [PubMed] [Google Scholar]
- 48.Akhter A, Gavrilin MA, Frantz L, Washington S, Ditty C, Limoli D, Day C, Sarkar A, Newland C, Butchar J, Marsh CB, Wewers MD, Tridandapani S, Kanneganti TD, Amer AO. Caspase-7 activation by the Nlrc4/Ipaf inflammasome restricts Legionella pneumophila infection. PLoS Pathog. 2009;5:e1000361. doi: 10.1371/journal.ppat.1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanjuan MA, Milasta S, Green DR. Toll-like receptor signaling in the lysosomal pathways. Immunol Rev. 2009;227:203–220. doi: 10.1111/j.1600-065X.2008.00732.x. [DOI] [PubMed] [Google Scholar]
- 50.Delgado MA, Elmaoued RA, Davis AS, Kyei G, Deretic V. Toll-like receptors control autophagy. EMBO J. 2008;27:1110–1121. doi: 10.1038/emboj.2008.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi CS, Kehrl JH. MyD88 and Trif target Beclin 1 to trigger autophagy in macrophages. J Biol Chem. 2008;283:33175–33182. doi: 10.1074/jbc.M804478200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanjuan MA, Dillon CP, Tait SW, Moshiach S, Dorsey F, Connell S, Komatsu M, Tanaka K, Cleveland JL, Withoff S, Green DR. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature. 2007;450:1253–1257. doi: 10.1038/nature06421. [DOI] [PubMed] [Google Scholar]
- 53.Saitoh T, Fujita N, Jang MH, Uematsu S, Yang BG, Satoh T, Omori H, Noda T, Yamamoto N, Komatsu M, Tanaka K, Kawai T, Tsujimura T, Takeuchi O, Yoshimori T, Akira S. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature. 2008;456:264–268. doi: 10.1038/nature07383. [DOI] [PubMed] [Google Scholar]
- 54.Andrade RM, Wessendarp M, Gubbels MJ, Striepen B, Subauste CS. CD40 induces macrophage anti-Toxoplasma gondii activity by triggering autophagy-dependent fusion of pathogen-containing vacuoles and lysosomes. J Clin Invest. 2006;116:2366–2377. doi: 10.1172/JCI28796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–766. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 56.Ling YM, Shaw MH, Ayala C, Coppens I, Taylor GA, Ferguson DJ, Yap GS. Vacuolar and plasma membrane stripping and autophagic elimination of Toxoplasma gondii in primed effector macrophages. J Exp Med. 2006;203:2063–2071. doi: 10.1084/jem.20061318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Birmingham CL, Smith AC, Bakowski MA, Yoshimori T, Brumell JH. Autophagy controls Salmonella infection in response to damage to the Salmonella-containing vacuole. J Biol Chem. 2006;281:11374–11383. doi: 10.1074/jbc.M509157200. [DOI] [PubMed] [Google Scholar]
- 58.Hernandez LD, Pypaert M, Flavell RA, Galan JE. A Salmonella protein causes macrophage cell death by inducing autophagy. J Cell Biol. 2003;163:1123–1131. doi: 10.1083/jcb.200309161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Amer AO, Swanson MS. Autophagy is an immediate macrophage response to Legionella pneumophila . Cell Microbiol. 2005;7:765–778. doi: 10.1111/j.1462-5822.2005.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Py BF, Lipinski MM, Yuan J. Autophagy limits Listeria monocytogenes intracellular growth in the early phase of primary infection. Autophagy. 2007;3:117–125. doi: 10.4161/auto.3618. [DOI] [PubMed] [Google Scholar]
- 61.Checroun C, Wehrly TD, Fischer ER, Hayes SF, Celli J. Autophagy-mediated reentry of Francisella tularensis into the endocytic compartment after cytoplasmic replication. Proc Natl Acad Sci USA. 2006;103:14578–14583. doi: 10.1073/pnas.0601838103. [DOI] [PMC free article] [PubMed] [Google Scholar]