Fig. 1.
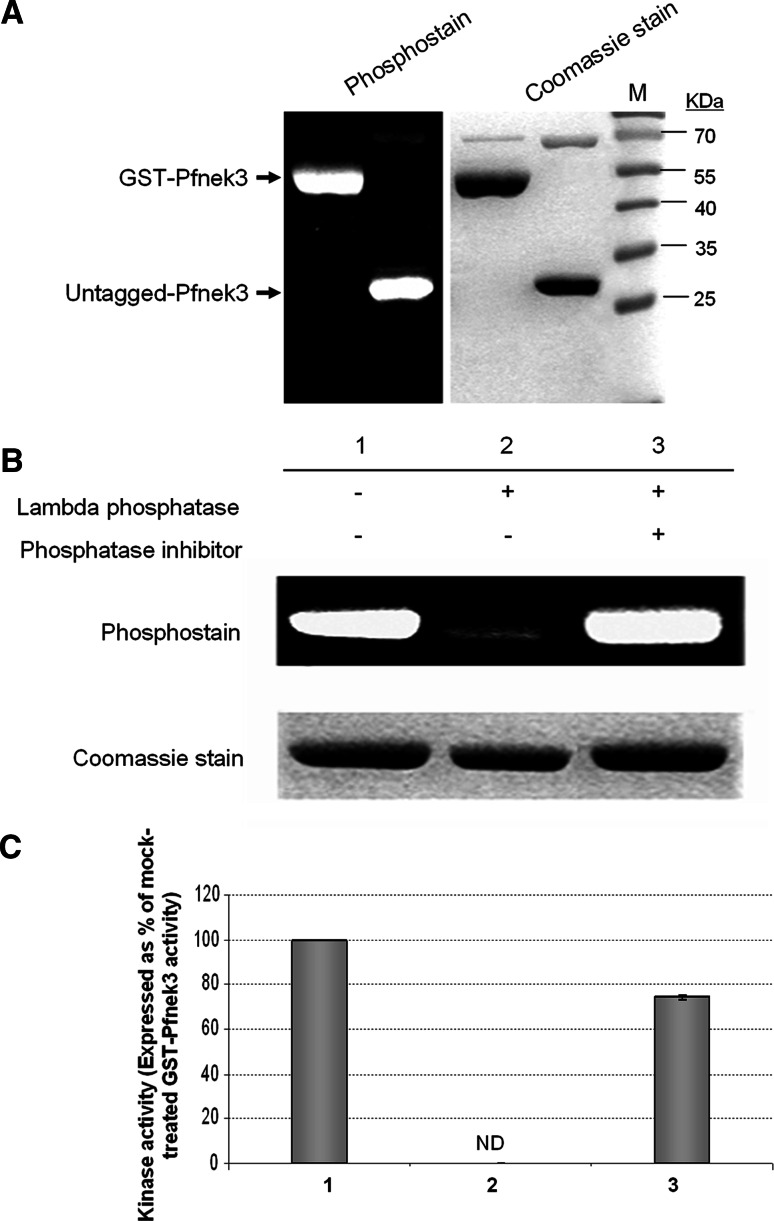
Activation of Pfnek3 is correlated with its phosphorylation status. a To authenticate the autophosphorylation ability of Pfnek3, GST-Pfnek3 was subjected to PreScission protease cleavage to remove the GST moiety. The phosphorylation level of the untagged recombinant kinase was subsequently analyzed using the Pro-Q Diamond phosphoprotein gel stain. The untagged Pfnek3 displayed a similar level of phosphorylation as its GST-fused counterpart. Lane M denotes the protein standard marker (Fermentas) used for estimating the protein sizes. b Purified GST-Pfnek3 was treated with lambda phosphatase in the presence (lane 3) or absence (lane 2) of the phosphatase inhibitor cocktail. In the mock-treated sample (lane 1), lambda phosphatase was omitted. c Following lambda phosphatase treatment in (b), an aliquot of the respective reaction mixture was transferred to MBP-coated microtiter plates to assay for the activity of dephosphorylated GST-Pfnek3. ND Kinase activity not detected