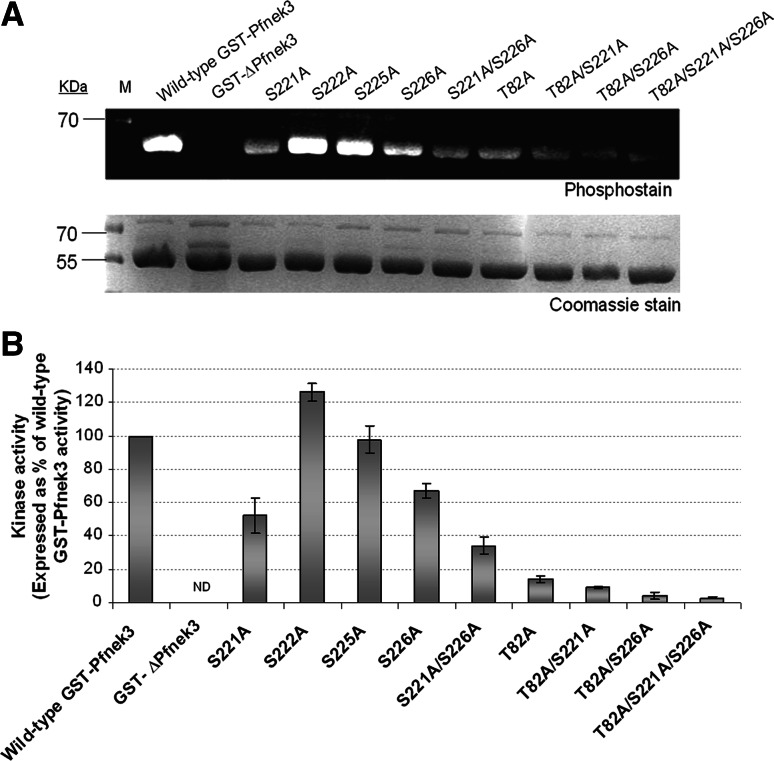
Fig. 3.
Autophosphorylation levels and kinase activities of GST-Pfnek3 alanine-substituted mutants. a Purified wild-type GST-Pfnek3 and the indicated mutants were resolved by SDS-PAGE and stained with the phosphoprotein gel stain to assess their autophosphorylation levels. In the protein standard marker (M), the 70-kDa protein band corresponds to a phosphoprotein that could be detected using the phosphostain. Following phosphostaining, the gel was stained with Coomassie Blue to ensure that relatively equal amounts of proteins were loaded. b The in vitro kinase activities of the respective GST-Pfnek3 mutants were subsequently determined by their ability to phosphorylate the MBP substrates. ND Kinase activity not detected