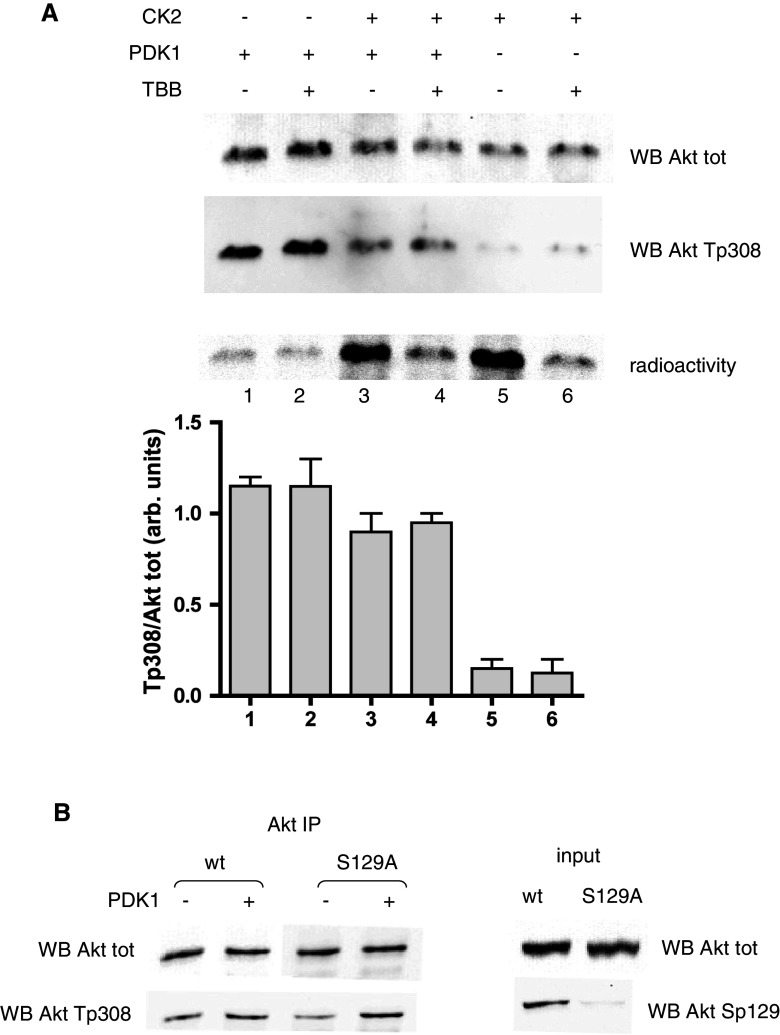
Fig. 2.
Effect of CK2 on PDK1 activity towards Thr308. a Recombinant ΔPH-Akt (240 ng) was pre-incubated for 40 min with a radioactive phosphorylation mixture in the presence or in the absence of CK2α (200 ng). Where indicated, TBB (10 μM) was added at the beginning of the radioactive reaction. After pre-incubation, a new nonradioactive mixture was added, together with PDK1 (20 ng) where indicated. Incubations were prolonged for an additional 10 min. Analysis was performed by SDS-PAGE followed by Western blot with the indicated antibodies; since incubation in the presence of CK2 turned out to reduce the amount of total Akt, the results are also shown as as a bar graph for Thr308 signal normalized to total Akt. Radioactivity was detected by electronic autoradiography, then radioactive bands were cut and counted by liquid scintillation; the CK2-dependent phosphorylation degree (lanes 3 and 5) was estimated at 18%. Three determinations were performed for each experiment; mean values ± SEM are shown. b HEK 293T cells were transfected with w.t. Akt or Ser129Ala Akt mutant, Akt was immunoprecipitated from cell lysates, and the washed pellets were incubated with a phosphorylation mixture, in the presence or in the absence of PDK1 (80 ng). Samples were analyzed by Western blot with the indicated antibodies; on the right (input), the Western blot analysis of 10 μg of total lysate proteins is shown