Abstract
The interaction of ankyrin and spectrin yields the major anchor between the membrane skeleton and the lipid bilayer. It is critical for red cell deformability and stability, and it is also involved in the cellular localization of several proteins, in cell differentiation, and in neuron activity. Therefore, its nature is of great interest, and recently, several researchers have had varying degrees of success in elucidating the structural basis of ankyrin–spectrin recognition. In this short paper, we briefly summarize the data obtained and compare the resulting conclusions.
Keywords: Spectrin, Ankyrin, Membrane skeleton, Protein structure, Protein–protein interactions
Why the membrane skeleton is so important
The remarkable structural and mechanical properties of the erythrocyte membrane come from the presence of a regular, multiprotein network called the membrane skeleton on its cytoplasmic surface. This is a unique arrangement of spectrin, ankyrin, actin, protein 4.1, and other proteins (Fig. 1), with direct and indirect connections to the membrane [1]. Ubiquitous in most metazoan cells as a polyfunctional organizing membrane scaffold, the membrane skeleton, in which spectrin plays the key role, is thought to be responsible for membrane stability, cell shape regulation and reversible deformation, organization of membrane components, recruitment to the cytoplasmic membrane surface of signaling and structural components, and guided trafficking of vesicles and organelles [2, 3].
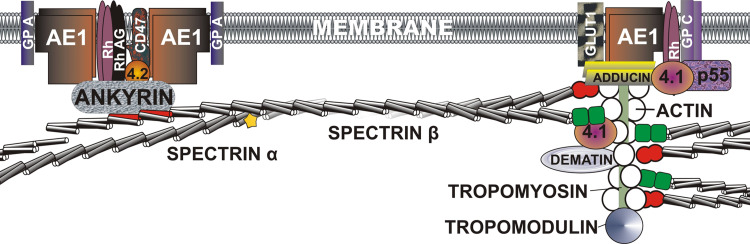
Fig. 1.
A model of the human red cell membrane. The network of the membrane skeleton is anchored to the plasma membrane via transmembrane proteins and through direct interactions with lipids [1, 11]. The domains of spectrin are: triple helical domain (black–white cylinders; ankyrin binding elements in red); CH domain (green squares); EF-hands (red merged circles); and SH3 domain (yellow star). AE1 anion exchanger 1, GLUT1 glucose transporter 1, GP A glycophorin A, GP C glycophorin C, Rh rhesus factor, Rh AG Rh antigen
Ankyrins form the major link between the membrane-spanning proteins and the underlying spectrin-based membrane skeleton. It is essential that ankyrin and spectrin remain in contact with each other during normal cellular processes, as reflected by the fact that defects in either the ankyrins or the spectrins disrupt membrane linkages, giving rise to a variety of diseases, as seen in hereditary conditions like hemolytic disease, cardiac arrhythmia, and spinocerebellar ataxia [4]. Thus, understanding the atomic basis for the interactions between spectrin and ankyrin became an extremely important problem to solve. Although many attempts had been made to create an unequivocal picture of the ankyrin-binding activity of erythroid β-spectrin, it was only a few months ago that several groups of researchers managed to throw light on it. The question remains whether we have a complete view of the issue, or if some matters have yet to be elucidated.
Spectrin repeats are intriguing, and some of them are extraordinary
Spectrin is a high molecular weight, flexible, rod-like protein formed by the head-to-head association of two antiparallel heterodimers of α (280 kDa) and β (247–460 kDa) subunits. In general, spectrin and spectrin-related proteins (e.g., dystrophin, α actinin, and plectin) share three major structural elements: a calmodulin-like domain containing EF-hands; CH (calponin homology) domains; and an approx. 106-amino acid residue repeat segments [5]. Structural studies on various triple-helical units of spectrin show that these are slightly distorted, left-handed coiled coils, where two helices are parallel (marked as A and C) and one is antiparallel (marked as B). The stability of the spectrin triple-helical repeats is assumed to be mostly dependent on inter-helical contacts, and the repeats are connected to one another by an ordered continuous helix [6, 7]. Subunit α contains 21 such units, including a single helix representing helix C of the partial repeat of the tetramerization region. The segment α10 differs from the others by an approx. 60-residue long insertion forming an SH3 domain. Conventional β-spectrins contain 17 repeat units together with helices A and B as part of the above-mentioned tetramerization repeat. βHeavy chains of spectrin comprise 30 repeats. The C-terminus of β-spectrins contains multiple phosphorylation sites, but some of the isoforms contain a PH domain. Until recently, spectrin repeats were regarded as modules which build extended molecules, which are responsible predominantly for structural and mechanical properties. Some spectrin repeats have been observed to have a high specificity of interactions with ligands such as proteins or phospholipids, so it can be deduced that the repeating units have evolved to provide functional specialization without changes to the conserved three-dimensional fold. This is reflected both by the comparison of spectrin repeats from distantly related organisms [8], and by the 25–30% amino acid sequence identity among repeats within the spectrin molecule [9]. Today, it is widely accepted that such modules also serve as a docking surface for cytoskeletal and signal transduction proteins and membrane elements [10, 11]. How can we explain the ligand specificity derived from regions with such a regular repetitive structure? This issue has been most thoroughly explored for the ankyrin-binding site located on the β chain of spectrin, which includes highly conserved regions within the 14th and 15th repeats of the protein (amino acid residues 1768–1898) [12, 13]. However, this stretch of the sequence does not display any obvious features that might distinguish it from other spectrin-repeating units. Discovering the ankyrin-sensitive binding site for PE-rich lipids in an N-terminal fragment of the domain makes this region of spectrin even more intriguing [14].
The ankyrin-binding tandem
The first step to enrich the rough and incomplete picture of the structure of the above-mentioned fragment of spectrin was to employ the site-directed spin labeling technique [15]. The results indicated that the lipid-binding domain of β-spectrin possesses a helical structure and is entirely composed of helix C of the 14th repeat unit. Most interestingly, the highly amphipathic character of the helix apparently correlates with its 310 configuration at its N-terminus. Although the crystal structures described below do not confirm the existence of such a configuration, it should be stressed that the structure of the N-termial region of helix C in at least some of the spectrin repeats has been found to be very flexible in solution [16]. This flexibility may barely be demonstrable in the static protein structures in crystals. Further studies confirmed the structural features of the whole 14th segment as typical for a triple-helical spectrin repeat, where the hydrophobic side chains of the amphipathic helix C are involved in core formation, and the charged side chains are exposed to the exterior [17]. It became clear that the observed clustering of negatively charged side chains on one side of the domain is not a coincidence, especially if it constrains the unusual conformation of the helix. It is very probably involved in the docking of other proteins or ligands, and ankyrin is the most suspected. This hypothesis found confirmation in the crystal structure of the 14th and 15th repeats of β-spectrin. Although there are three crystal structures for the ankyrin-binding domain available thanks to the recent effort of independent teams (PDB IDs: 3EDV, 3F57, 3EDU), and it has consistently been pointed out that there are similarities to all the other spectrin repeats [18–20], not all the authors considered the patch of highly conservative anionic amino acid residues on the 14th repeat (E1764, E1770, D1773, E1777, D1781, E1784, D1787) to be a docking site for ankyrin. Ipsaro et al. [19] matched it up for charge and shape complementarities with cationic amino acid residues found in the separately crystallized spectrin-binding fragment of ankyrin, and suggested that there is no obvious structural need for the 15th repeat. By contrast, Stabach et al. [20] mutated selected residues of the fragment of β-spectrin, which led them to identify 14 amino acid residues crucial for ankyrin binding, the most critical of which are D1781, E1784, D1787, T1788, L1792, Y1866 and A1867. It is worth stressing that several of the identified residues coincided with those hypothesized previously, and that the others were found either within the linker between the two repeats or on a loop flanked by helices B and C of repeat 15. Davis et al. [18] postulated that the latter structural element forms an ankyrin-binding site of β-spectrin, but in their mutagenesis experiments, they skipped over the negatively charged cluster. However, the above-mentioned loop, including the highly conserved motif AYA1867, seems to play a major role in controlling the tilt angle between the 14th and 15th repeats of β-spectrin via its interactions with the side chains of the adjacent linker [20], and this unusual bend emerged as a distinct feature of the two-repeat crystal structure that binds ankyrin. By contrast, Ipsaro et al. [19] observed a significant flexibility of this region, but initially they ruled out the possibility that the tandem repeats assume an unusual arrangement which could explain its specific recognition by ankyrin. The 14th and 15th repeats of β-spectrin definitely appeared to be the tandem repeats necessary for the ankyrin-binding activity of spectrin, which is reflected by their binding affinity being similar to that of the intact complex [13]. While electrostatic interactions via a number of negatively charged residues within repeat 14 and the following linker region seem necessary to form a stable spectrin–ankyrin complex, they may not be sufficient. In this way, the cluster of acidic amino acid residues with contributions from the downstream linker and the loop spanning helices B and C of the 15th repeat may form a flexible pocket on β-spectrin that traps ankyrin. How such a scenario was confirmed is described at the end of this paper.
ZU5 domain: a novel structural motif
Ankyrins are proteins expressed in a great variety of tissue-specific isoforms in humans, with a modular arrangement consisting of an N-terminal membrane-binding domain, a spectrin-binding domain, and a C-terminal regulatory domain. Although many cellular functions of human erythroid ankyrin (marked as ankyrin R) have been studied, until recently structural work on this protein has been largely restricted to the N-terminal domain, which is composed of 24 L-shaped tandem repeats that mediate attachment to the membrane through interactions with membrane-embedded proteins (e.g., anion exchanger) [2]. The minimal spectrin-binding region was identified as a ZU5 domain together with the following 55 residues of ankyrin R [13, 21]. Common features of proteins containing ZU5 include their location near the cell membrane and their high affinity interactions with other proteins. Ipsaro et al. [19] were the first to describe the structure of the spectrin-binding motif of ankyrin R. They indicated a compact ZU5 domain consisting of a β-strand core and some surface loops together with two short helices and two strands contributing to the core formation (PDB ID: 3KBT). They also identified a small patch of positively charged residues on one face of the structure that are highly conserved in ankyrins. This positive charge suggests its role in spectrin binding via charge-charge interactions. It has been suggested that the spectrin-binding domain is the first of two consecutive ZU5 domains present in ankyrin and that the second one may interact with the Unc5-PIDD-Ankyrin (UPA) and Death domains of the protein, which correspond to the recently described supramolecular nature of the cytoplasmic portion of the UNC5b netrin receptor [22]. It is worth emphasizing that both the ZU5 domains of ankyrin retain putative binding surfaces for multiple inter- and intramolecular contacts.
The complex structure: is it dynamic or fixed?
Concerning the dynamic behavior of the membrane skeleton and of spectrin in particular, static crystal structures of separate proteins might not be sufficient to elucidate the true nature of ankyrin–spectrin contacts. As shown previously, the structure of the ankyrin-dependent lipid-binding site of β-spectrin is flexible, and it undergoes significant changes at the level of its spatial arrangement, i.e., partial opening of the coiled-coil structure of the whole 14th repeat upon interaction with phospholipids and detergents [17, 23]. This is also true in other proteins consisting of spectrin-like repeats [24]. On the other hand, the most recent structural data provided by Ipsaro and Mondragon [25], showing the crystal structure of the 13th to 15th β-spectrin repeats in complex with the spectrin-binding domain of human ankyrin R (PDB ID: 3KBT), suggests that the recognition of ankyrin by spectrin requires shape complementarity, but no induced fit takes place. The ZU5 core β sheets are laid down atop a great portion of the 14th repeat parallel to the long axis of spectrin and are docked at the B–C loop in repeat 15. In fact, the inter-repeat kink seems to be a hallmark for ankyrin-binding tandem segments of spectrin, even in a non-bound state. This might be the key determinant underlying the attraction of ankyrin by spectrin [26], but in the light of the recent data on erythrocyte spectrin tetramerization domain complex, a bend of approximately 50° observed between 14th and 15th repeats may also be an attribute of other spectrin repeats [27]. It should be stressed that interactions between the two proteins are both charge–charge interactions and van der Waals and hydrogen bonds, and that the majority of the residues involved in the binding interface are highly conserved [25]. The roles of the residues deduced from the crystal structure of the complex were further confirmed by binding analyses. As a rule, the closer a negatively charged residue is to the linker between the 14th and 15th spectrin repeats, the stronger its participation in ankyrin binding seems to be. However, at least one such residue was found outside the C-terminal end of helix C of the 14th repeat (E1710). Going further, spectrin contacts ankyrin tightly via interactions of Y1866 within the B–C loop of the 15th repeat, with the latter stabilized by A1867 [25, 26].
A key question is how ankyrin maintains its interaction with spectrin during mechanical stress, when spectrin repeats reversibly undergo forced unfolding. In such conditions, the spectrin is still attached to membranes [28], so the ankyrin linkage to the double repeat spectrin unit is supposed to remain intact. Davis et al. [18] speculate that, since the region of a loop flanked by helices B and C of spectrin repeats is well preserved in forced unfolding simulations, the ankyrin bound to that loop maintains its interaction with spectrin even when the 15th repeat is in a non-native conformation. On the other hand, Stabach et al. [20] suggest a mechanism of transduction of mechanical stress into alterations in ligand binding which may provide controlled plasticity of membrane scaffold and a mechanical sensor controlling the arrangement of ligands or signal-transducing elements in response to membrane deformation. This is supported by the fact that both the observed bend between the 14th and 15th repeats of spectrin and their cooperative folding are required for high-affinity ankyrin binding. Of course, it may be possible that the ankyrin-binding site remains intact during extension, while the non-bound helices may preferentially unwind and absorb the extension force. This concurs with the observations that the clinical mutations found near the ankyrin-binding site do not exert a significant effect on the binding affinity but rather cause folding instability [25]. Each of the depicted scenarios is attractive and highly probable, but they need to be verified.
Conclusions
Despite the overall structural constrains common for all spectrin repeats, this type of structural motifs has been evolutionally tailored to interact with an array of binding partners. Considering all the data given above, it emerges that the presence of a highly acidic patch and some other highly conserved residues together with spatial arrangement of those elements distinguish the tandem (14th and 15th repeat) from the other spectrin segments, and that these features contribute to the formation of a pocket on β spectrin that associates with ankyrin. Thus, we now have the first complete picture of the spectrin repeats in the complex with the binding molecule, as well as the earliest example of the ZU5 intermolecular complex. However, the dynamic aspects of the complex have yet to be elucidated. Now it is time to employ such a strategy with other spectrin repeats, as particular arrangements of tandem spectrin segments exposing unique patches of charged and/or hydrophobic residues could build up a diverse set of specific molecular epitopes matching a variety of distinct ligands.
Acknowledgements
This publication was possible thanks to the financial support of the Foundation for Polish Science. We acknowledge Mr. Derek Handley for proofreading this manuscript.
References
- 1.Anong WA, Franco T, Chu H, Weis TL, Devlin EE, Bodine DM, An X, Mohandas N, Low PS. Adducin forms a bridge between the erythrocyte membrane and its cytoskeleton and regulates membrane cohesion. Blood. 2009;114:1904–1912. doi: 10.1182/blood-2009-02-203216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett V, Baines AJ. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol Rev. 2001;81:1353–1392. doi: 10.1152/physrev.2001.81.3.1353. [DOI] [PubMed] [Google Scholar]
- 3.DeMatteis MA, Morrow JS. Spectrin tethers and mesh in the biosynthetic pathway. J Cell Sci. 2000;113:2331–2343. doi: 10.1242/jcs.113.13.2331. [DOI] [PubMed] [Google Scholar]
- 4.Bennett V, Healy J. Organizing the fluid membrane bilayer: diseases linked to spectrin and ankyrin. Trends Mol Med. 2008;14:28–36. doi: 10.1016/j.molmed.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Broderick MJF, Winder SJ. Towards a complete atomic structure of spectrin family proteins. J Struct Biol. 2002;137:184–193. doi: 10.1006/jsbi.2002.4465. [DOI] [PubMed] [Google Scholar]
- 6.Kusunoki H, Minasov G, MacDonald RI, Mondragón A. Independent movement, dimerization and stability of tandem repeats of chicken brain a-spectrin. J Mol Biol. 2004;344:495–511. doi: 10.1016/j.jmb.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 7.An X, Guo X, Zhang X, Baines AJ, Debnath G, Moyo D, Salomao M, Bhasin N, Johnson C, Discher D, Gratzer WB, Mohandas N. Conformational stabilities of the structural repeats of erythroid spectrin and their functional implications. J Biol Chem. 2006;281:10527–10532. doi: 10.1074/jbc.M513725200. [DOI] [PubMed] [Google Scholar]
- 8.Baines A. Comprehensive analysis of all triple helical repeats in beta-spectrins reveals patterns of selective evolutionary conservation. Cell Mol Biol Lett. 2003;8:195–214. [PubMed] [Google Scholar]
- 9.Leluk J, Hanus-Lorenz B, Sikorski AF. Application of genetic semihomology algorithm to theoretical studies on various protein families. Acta Biochim Pol. 2001;48:21–33. [PubMed] [Google Scholar]
- 10.Djinovic-Carugo K, Gautel M, Ylänne J, Young P. The spectrin repeat: a structural platform for cytoskeletal protein assemblies. FEBS Lett. 2002;513:119–123. doi: 10.1016/S0014-5793(01)03304-X. [DOI] [PubMed] [Google Scholar]
- 11.Sikorski AF, Czogalla A, Hryniewicz-Jankowska A, Bok E, Plażuk E, Diakowski W, Chorzalska A, Kolondra A, Langner M, Grzybek M (2008) Interactions of erythroid and nonerythroid spectrins and other membrane-skeletal proteins with lipid mono- and bilayers. In: Leitmannova LA (ed) Advances in Planar Lipid Bilayers and Liposomes, vol 6. Elsevier, New York, pp 81–102
- 12.Kennedy SP, Warren SL, Forget BG, Morrow JS. Ankyrin binds to the 15th repetitive unit of erythroid and nonerythroid beta-spectrin. J Cell Biol. 1991;115:267–277. doi: 10.1083/jcb.115.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ipsaro JJ, Huang L, Gutierrez L, MacDonald RI. Molecular epitopes of the ankyrin-spectrin interaction. Biochemistry. 2008;47:7452–7464. doi: 10.1021/bi702525z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hryniewicz-Jankowska A, Bok E, Dubielecka P, Chorzalska A, Diakowski W, Jezierski A, Lisowski M, Sikorski AF. Mapping of an ankyrin-sensitive, phosphatidylethanolamine/phosphatidylcholine mono- and bi-layer binding site in erythroid beta-spectrin. Biochem J. 2004;382:677–685. doi: 10.1042/BJ20040358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Czogalla A, Jaszewski AR, Diakowski W, Bok E, Jezierski A, Sikorski AF. Structural insight into an ankyrin-sensitive lipid-binding site of erythroid β-spectrin. Mol Membr Biol. 2007;24:215–224. doi: 10.1080/09687860601102427. [DOI] [PubMed] [Google Scholar]
- 16.Grum VL, Li D, MacDonald RI, Mondragon A. Structures of two repeats of spectrin suggest models of flexibility. Cell. 1999;98:523–535. doi: 10.1016/S0092-8674(00)81980-7. [DOI] [PubMed] [Google Scholar]
- 17.Czogalla A, Grzymajło K, Jezierski A, Sikorski AF. Phospholipid-induced structural changes to an erythroid beta spectrin ankyrin-dependent lipid-binding site. Biochim Biophys Acta. 2008;1778:2612–2620. doi: 10.1016/j.bbamem.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 18.Davis L, Abdi K, Machius M, Brautigam C, Tomchick DR, Bennett V, Michaely P. Localization and structure of the ankyrin-binding site on beta2-spectrin. J Biol Chem. 2009;284:6982–6987. doi: 10.1074/jbc.M809245200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ipsaro JJ, Huang L, Mondragón A. Structures of the spectrin-ankyrin interaction binding domains. Blood. 2009;113:5385–5393. doi: 10.1182/blood-2008-10-184358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stabach PR, Simonović I, Ranieri MA, Aboodi MS, Steitz TA, Simonović M, Morrow JS. The structure of the ankyrin-binding site of beta-spectrin reveals how tandem spectrin-repeats generate unique ligand-binding properties. Blood. 2009;113:5377–5384. doi: 10.1182/blood-2008-10-184291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolondra A, Grzybek M, Chorzalska A, Sikorski AF. The 22,5 kDa spectrin-binding domain of ankyrinR binds spectrin with high affinity and changes the spectrin distribution in cells in vivo. Protein Expr Purif. 2008;60:157–164. doi: 10.1016/j.pep.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Wang R, Wei Z, Jin H, Wu H, Yu C, Wen W, Chan L-N, Wen Z, Zhang M. Autoinhibition of UNC5b revealed by the cytoplasmic domain structure of the receptor. Mol Cell. 2009;33:692–703. doi: 10.1016/j.molcel.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 23.Paździor G, Chorzalska A, Czogalla A, Borowik T, Sikorski AF, Langner M. Fluorescence approach to evaluating conformational changes upon binding of β-spectrin ankyrin-binding domain mutants with the lipid bilayer. Gen Physiol Biophys. 2009;28:283–293. doi: 10.4149/gpb_2009_03_283. [DOI] [PubMed] [Google Scholar]
- 24.Legardinier S, Raguénès-Nicol C, Tascon C, Rocher C, Hardy S, Hubert JF, Le Rumeur E. Mapping of the lipid-binding and stability properties of the central rod domain of human dystrophin. J Mol Biol. 2009;389:546–558. doi: 10.1016/j.jmb.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 25.Ipsaro JJ, Mondragón A (2010) Structural basis for spectrin recognition by ankyrin. Blood Doi: 10.1182/blood-2009-11-255604 [DOI] [PMC free article] [PubMed]
- 26.La-Borde PJ, Stabach PR, Siminović I, Morrow JS, Siminović M. Ankyrin recognizes both surface character and shape of the 14–15 di-repeat of β-spectrin. Biochem Biophys Res Commun. 2010;392:490–494. doi: 10.1016/j.bbrc.2010.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ipsaro JJ, Harper SL, Messick TE, Marmorstein R, Mondragón A, Speicher DW (2010) Crystal structure and functional interpretation of the erythrocyte spectrin tetramerization domain complex. Blood. doi:10.1182/blood-2010-01-261396 [DOI] [PMC free article] [PubMed]
- 28.Discher DE, Mohandas N, Evans EA. Molecular maps of red cell deformation: hidden elasticity and in situ connectivity. Science. 1994;266:1032–1035. doi: 10.1126/science.7973655. [DOI] [PubMed] [Google Scholar]