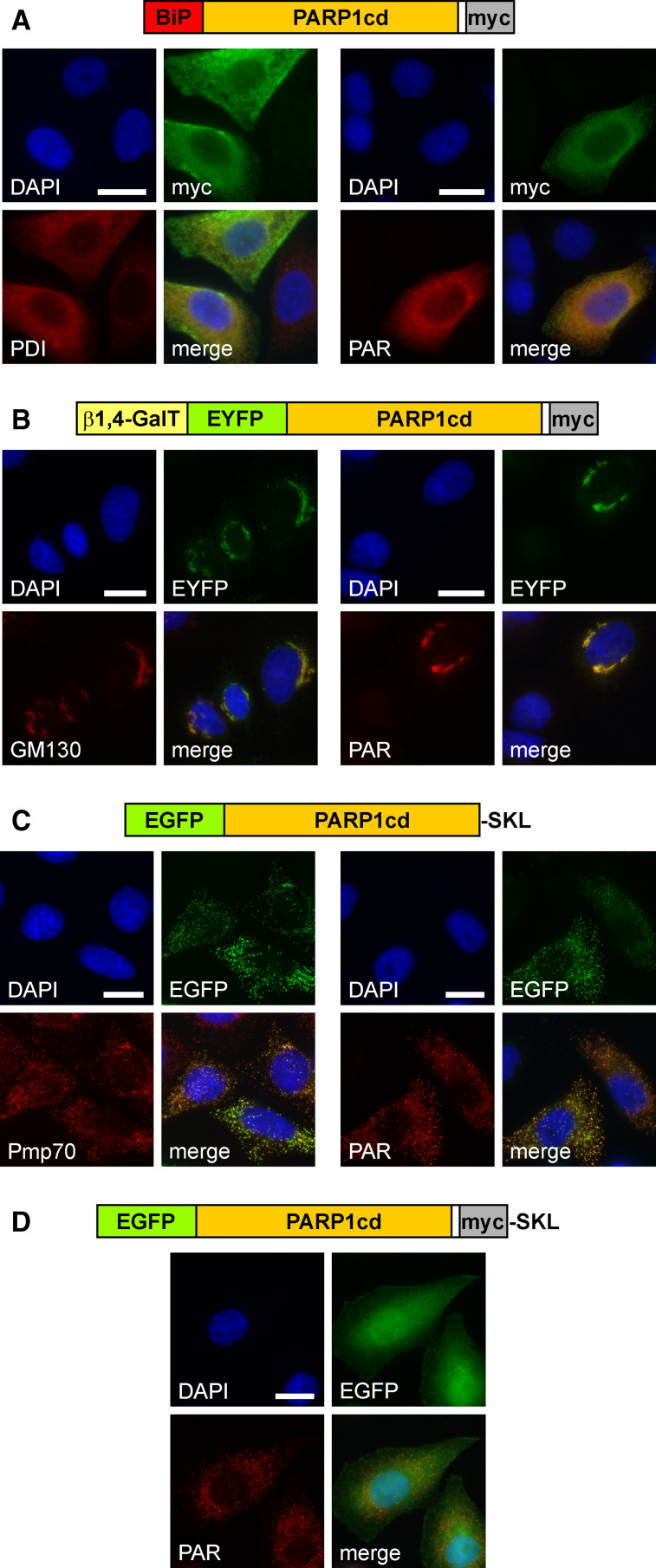
Fig. 4.
Targeted expression of PARP1cd to the lumen of the endoplasmic reticulum, the Golgi apparatus or peroxisomes results in detectable PAR formation within these organelles. a HeLa S3 cells transfected with a vector encoding the first 100 amino acids of binding immunoglobulin protein (BiP) N-terminally fused to PARP1cd (BiP-PARP1cd) were subjected to immunocytochemistry after 24 h. The overexpressed protein (myc) co-localized with the ER marker protein disulfide isomerase, PDI (left), and mediated PAR accumulation (right). Bar 10 μm. b HeLa S3 cells were transfected with a vector encoding a Golgi-targeted EYFP-PARP1cd fusion construct, gEYFP-PARP1cd. The intrinsic EYFP-fluorescence of the overexpressed recombinant protein localized to the Golgi complex as revealed by immunostaining of the Golgi-specific marker protein GM130 (left). PAR accumulation (right) was observed in cells overexpressing gEYFP-PARP1cd. Bar 10 μm. c HeLa S3 cells were transiently transfected with a vector encoding EGFP-PARP1cd harboring the C-terminal tripeptide SKL for peroxisomal targeting. Cells were subjected to immunocytochemistry after 24 h using antibodies against the peroxisomal marker protein Pmp70 (left). The overexpressed protein led to PAR accumulation within peroxisomes (right). Expression of the recombinant protein was monitored by its intrinsic EGFP fluorescence. Bar 10 μm. d An additional myc epitope, immediately preceding the C-terminal SKL signal, perturbed peroxisomal localization and led to predominant cytoplasmic distribution of the overexpressed protein. However, accumulation of PAR in peroxisomes revealed that the protein was still partially localized within the organelles