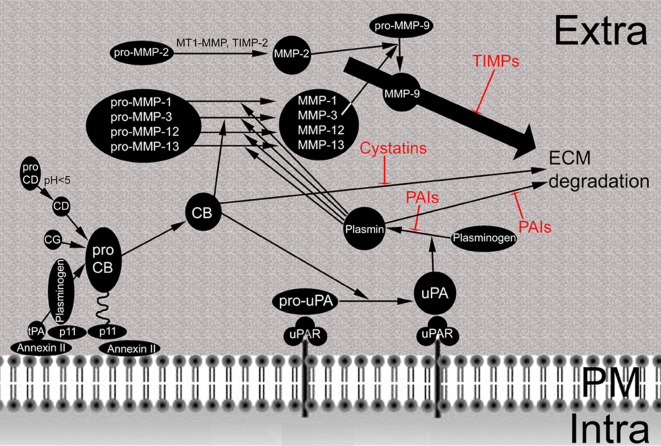
Fig. 2.
Protease network at the cellular surface of tumor cells. Pro-cathepsin (pro-CB) is attached via the annexin II/p11 heterotetramer to the plasma membrane: p11 binds pro-CB at one site and plasminogen at another; annexin II attaches the complex to the plasma membrane. Cathepsin D (CD), which is activated by acid pH, cathepsin G (CG) and tissue plasminogen activator (tPA) in the presence of plasminogen cleave pro-CB to active CB. Pro-urokinase plasminogen activator (uPA) is activated after binding to the urokinase plasminogen activator receptor (uPAR) by active CB. Thereafter, uPA activates plasminogen to plasmin. Plasmin cleaves pro-MMP-1, -3, -12 and -13 to generate the respective active enzymes. MMP-3 is also activated directly by CB. MMP-2 is activated by combined action of MT1-MMP and TIMP-2 (see Fig. 3 for detail) and activates MMP-9. Degradation of ECM by CB, plasmin and MMPs is inhibited by cystatins, PAIs and TIMPs, respectively. Extra Extracellular space, PM plasma membrane, Intra intracellular space