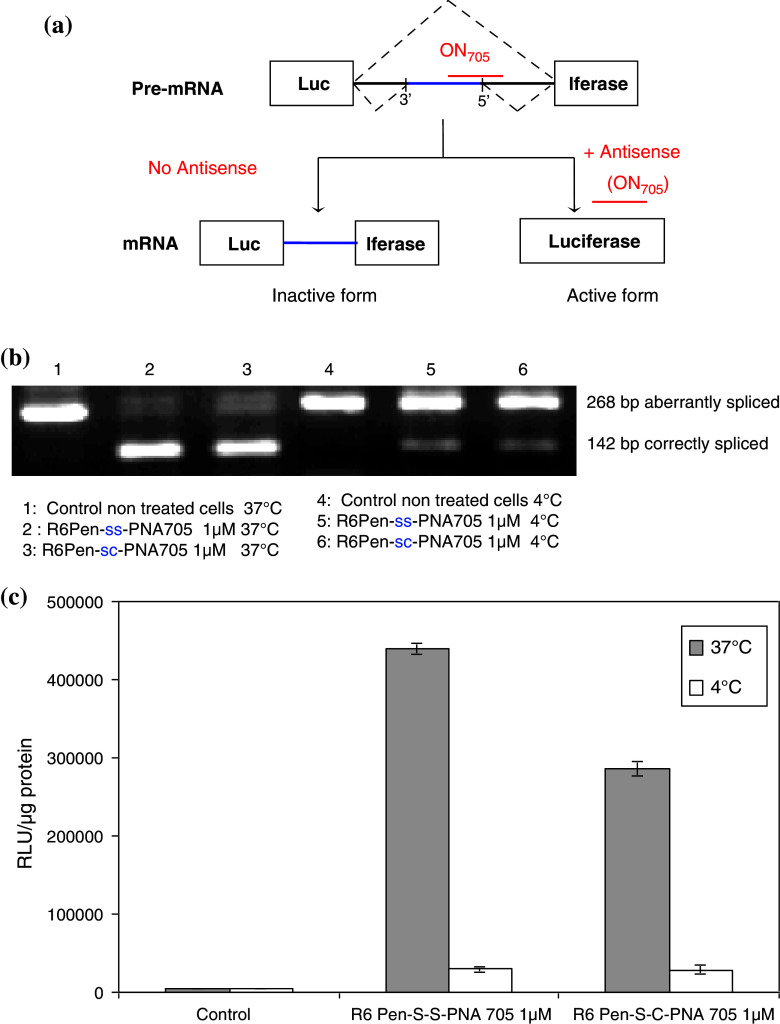
Fig. 1.
Splicing redirection assay. a A mutated β-globin gene intron carrying a cryptic splice site has been inserted in the coding sequence of a luciferase reporter gene and the construct has been stably transfected in HeLa pLuc 705 cells. Abnormal splicing does not allow the complete removal of the intron and no functional luciferase is produced. The nuclear delivery of a steric block ON analogue (ON 705) and its binding to the mutated site redirects the cellular splicing machinery towards complete elimination of this intron, leading to the production of the correctly spliced luciferase mRNA and to the production of active luciferase protein. b Luciferase up-regulation by PNA conjugates at low and high temperatures. HeLa pLuc 705 cells were preincubated for 30 min at 4 or 37°C before treatment. Cells were then incubated with 1 μM R6Pen-ss-PNA or R6Pen-sc-PNA in OptiMEM for 1 h at low or high temperatures and washed twice with PBS buffer. Luciferase quantification was processed after 23 h supplementary incubation in DMEM at 37°C. These two PNA conjugates differ in the nature of the linker between the peptidic and the oligonucleotidic parts of the conjugates (reducible disulfide—ss—or stable thioester—sc—covalent bond). c RT–PCR analysis of splicing redirection. Total RNA was purified from the same cellular lysates and amplified by RT–PCR. The correctly spliced mRNA can be distinguished from the aberrant one for each conjugate at the two temperatures