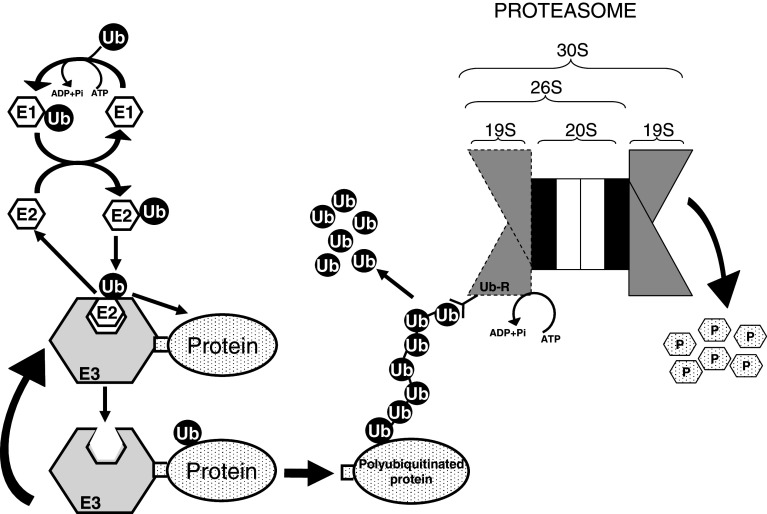
Fig. 3.
The ubiquitin-proteasome system is the major non-lysosomal pathway of intracellular proteolysis. The process has two parts: (1) substrate polyubiquitination (on the left) and degradation of the tagged protein by the downstream 26S/30S proteasome (on the right). Canonical ubiquitination involves three steps: (1) activation of ubiquitin by E1 enzyme in an ATP-dependent manner, (2) transfer of ubiquitin from E1 to an E2 enzyme, and (3) direct or indirect transfer of ubiquitin to a specific protein substrate recognized by an E3 enzyme. Further incorporation of other activated ubiquitin molecules generates a polyubiquitin chain. Although polyubiquitination is a reversible process, most proteins with polyubiquitin chains of four or more ubiquitins (Ub) attached to an inner Lys of the substrate and each other by their Lys48 are recognized by a subunit (Ub-R) on the 19S regulatory particle of the 26S/30S proteasome, deubiquitinated to generate free ubiquitin, and degraded in the 20S catalytic core to peptides (P) by processes that also consume ATP