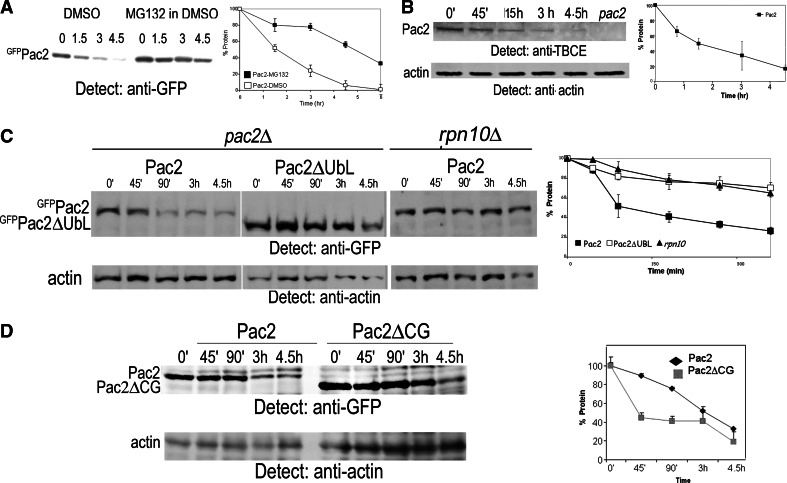
Fig. 4.
Turnover of Pac2 depends on interaction of its UbL domain with Rpn10. a Pac2 is stabilized in cells treated with the proteasome inhibitor MG132. pGAL-GFP-PAC2 transformed into pdr5Δ mutant cells was induced overnight with 2% galactose. Next morning the cells were diluted 1:3, and after 2 h 100 μl MG132 dissolved in DMSO was added (controls were treated with DMSO), together with 3% glucose to repress transcription and 10 mM CHX to inhibit translation. Equal aliquots of cells were analyzed at the zero time point and at the times indicated above each lane by anti-GFP immunoprecipitation followed by Western blotting. Graph shows protein remaining at each time point. b Half-life of endogenous Pac2 determined using an affinity-purified polyclonal antibody to human TBCE; 10 mM CHX was added at the zero time point, and equal aliquots were analyzed by TCA precipitation and Western blotting at the times indicated. Extract from pac2Δ mutants was used as a control for the specificity of the antibody; anti-actin was used as a loading control. c Pac2 and Pac2ΔUbL turnover in pac2Δ and rpn10Δ cells: pGAL-GFP-PAC2 or pGAL-GFP-PAC2ΔUBL were induced overnight in pac2Δ mutants, and pGAL-GFP-PAC2 was induced overnight in rpn10Δ mutants. At the zero time point, 3% glucose and 10 mM CHX were added, and equal aliquots of cells were TCA-precipitated for Western blotting at the times indicated above the lanes. Actin was used as a loading control. d Pac2 and Pac2ΔCG turnover in pac2Δ mutants: experimental conditions as in c