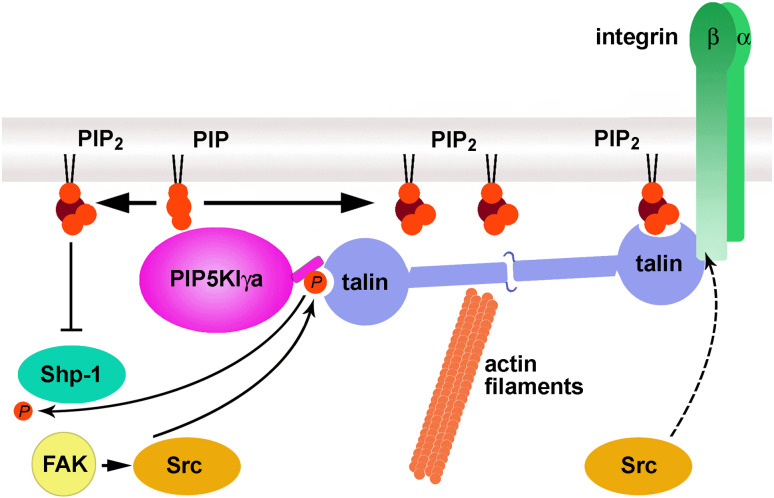
Fig. 4.
Talin and PIP5KIγa/c interact during focal contact assembly. Upon cell activation, PIP5KIγa/c undergoes phosphorylation on Tyr644 by Src kinase, which also associates with it. Both events are positively regulated by FAK kinase. When phosphorylated, PIP5KIγa/c binds to the FERM domain located in the globular N-terminal part of talin. The kinase produces PI(4,5)P2, which binds to the FERM domain of talin and relieves autoinhibitory interaction of the talin head with rod. At these conditions, talin is able to bind β-integrins, inducing additionally their aggregation and activation. Intracellularly, talin binds actin filaments, which enables the formation of stress fibers. The Tyr644 can be dephosphorylated by Shp-1, which associates with PIP5KIγ. The activity of Shp-1 is inhibited by PI(4,5)P2 favoring the lipid accumulation. On the other hand, the binding of β-integrin to talin can be inhibited by phosphorylation of β-integrin by Src kinase