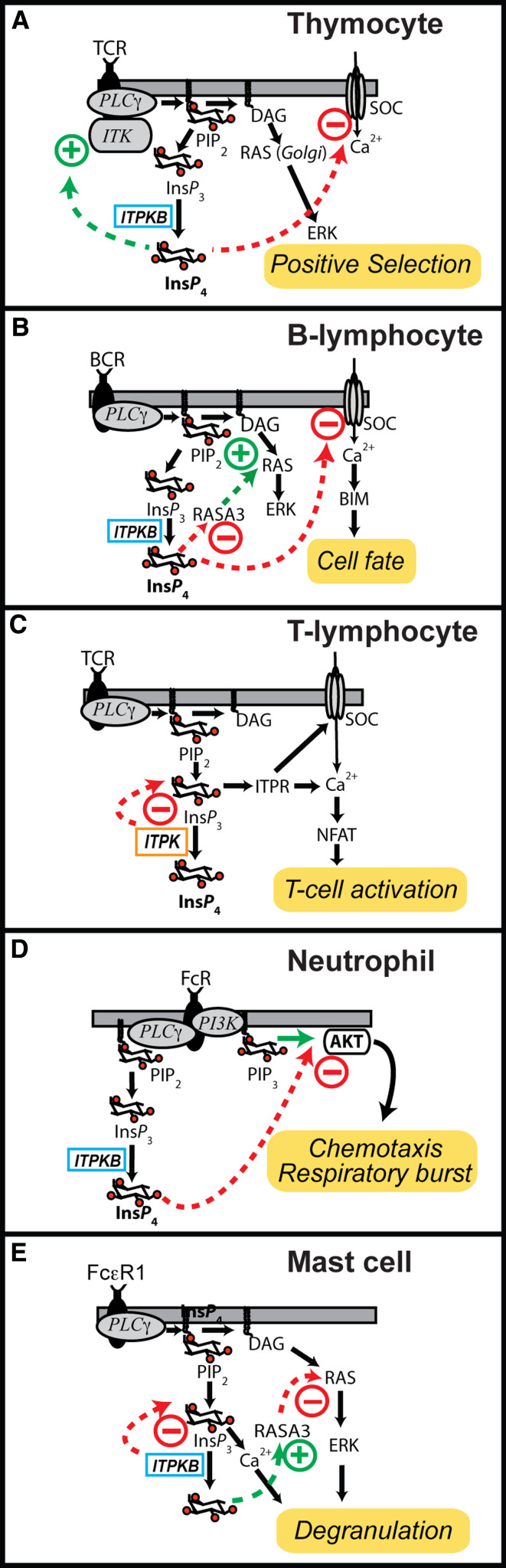
Fig. 4.
Diversity of ITPK signaling in immune cells. a Mice with targeted disruption in the gene for ITPKB exhibit major deficits in thymocyte maturation, with very few thymocytes undergoing positive selection [51, 52]. Activation of the T cell receptor in thymocytes derived from these mice results in less MAP kinase activation than in controls. At least two molecular mechanisms have been proposed to explain the phenotype. In one mechanism, Ins(1,3,4,5)P 4 triggers a feed-forward mechanism, whereby it binds and activates the interleukin-2 inducible T cell tyrosine kinase (ITK) [123] (green arrow). Downstream targets of ITK, such as phospholipase C gamma (PLCγ1), are thus hypoactive in the knockouts. A second mechanism proposes that store-operated Ca2+ channels (SOCs) are negatively regulated by Ins(1,3,4,5)P 4 (red arrow). In this model, lack of Ins(1,3,4,5)P 4 causes excessive Ca2+ influx, and this leads to deficits in Ca2+ homeostasis and its associated signaling [117]. b B lymphocytes from ITPKB knockout are anergic and undergo excessive apoptosis. Knockout B cells exhibit enhanced Ca2+ influx through SOCs, and this is attributed to a loss of normal inhibition on SOC by Ins(1,3,4,5)P 4 [117, 121] (red arrow). A second model suggests that Ins(1,3,4,5)P 4 is a negative regulator RASA3, a Ras GTPase activating protein that binds Ins(1,3,4,5)P 4 with high affinity [110] (red arrow). In this model, lack of Ins(1,3,4,5)P 4 leads to excessive GTPase activity, thus attenuating Ras and its downstream targets [110]. c Proposed role for ITPK signaling in mature, circulating T lymphocytes based on polymorphisms in the ITPKC gene linked to Kawasaki disease, an autoimmune disorder [59]. This model posits a hyperactive Ins(1,4,5)P 3-triggered Ca2+ response following activation of the T cell receptor (TCR), leading to excessive activation of the phosphatase calcineurin, causing dephosphorylation and over activation of the transcription factor NFAT. The hyperactive NFAT would result in an inappropriately large immune response in the vascular and mucosal systems, accounting for the Kawasaki disease phenotype. In contrast to the other immune models, no gain-of-function role for Ins(1,3,4,5)P 4 is invoked. d ITPKB also plays widespread roles in the innate immune system [120]. In neutrophils, Ins(1,3,4,5)P 4 generated downstream of Fc receptor (FcR) activation is proposed to compete with the head group of the lipid phosphatidylinositol (3,4,5)P3 (PIP3) for binding to the tyrosine kinase AKT. Thus, neutrophils derived from ITPKB knockouts exhibit enhanced PIP3-regulated responses, such as chemotaxis and the respiratory burst [53]. e Yet another mechanism is suggested to explain ITPKB signaling in mast cells [109]. Here, Fc receptor epsilon 1 (FcεR1) regulates degranulation via two ITPKB-dependent processes. The first is a straightforward reduction of Ca2+ release via ITPKB attenuation of Ins(1,4,5)P 3 signals (red arrow). The second, in contrast to the mechanism depicted in panel B, involves a positive regulation of RASA3 and concomitant negative effect on Ras activation status. In either mechanism shown in panel e, lack of ITPKB would produce hyperactive degranulation