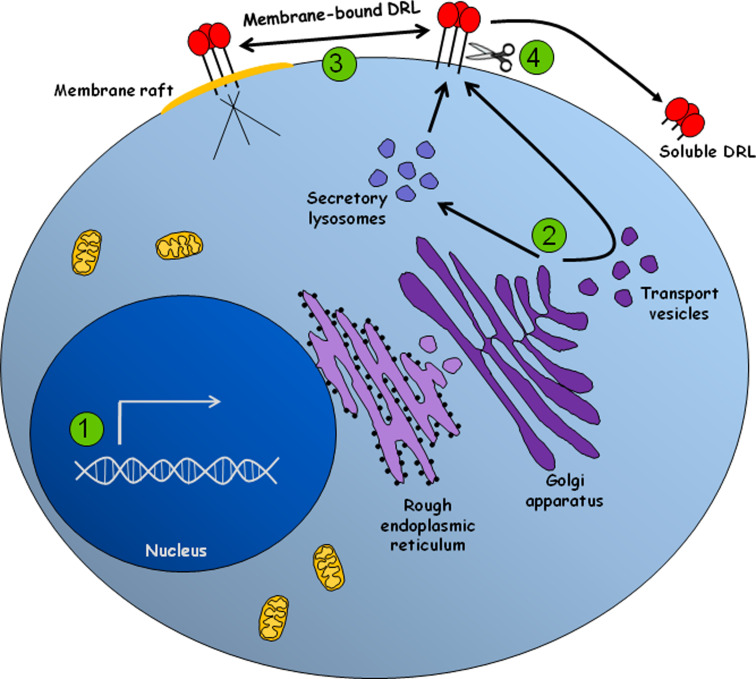
Fig. 1.
The expression of death receptor ligands is tightly modulated at different levels. 1 Transcription of death receptor ligands is modulated by groups of transcriptional activators or repressors that are activated and/or de novo expressed in response to a variety of stimuli. 2 FasL is either sorted directly to the plasma membrane or stored in secretory lysosomes. Sorting of FasL is dependent on its cytoplasmic PRD domain, which interacts with proteins that contain SH3 domains or WW motifs, including Nck, Grb2, PSTPIP and members of the PHC family of proteins. Similarly, TNF-α, which is normally delivered to the cell surface, can also be stored in secretory lysosomes. However, TNF-α storage depends on a brief exposure at the plasma membrane followed by re-endocytosis. TRAIL can also be found in secretory lysosomes although its sorting mechanism still remains to be elucidated. 3 Recruitment of FasL to membrane rafts boost its killing potential, probably by enhancing the clustering of Fas in target cells. Trafficking of FasL into the rafts is dependent on the cytoplasmic PRD domain of FasL and independent of the SxxS motif. To date, TRAIL and TNF-α redistribution to the membrane rafts is still unclear. 4 Once in the cell surface, death receptor ligands can be proteolytically shed, being converted into soluble molecules. The soluble forms of these ligands can acquire different or even opposing functions, including the induction of proliferation, inflammation and survival. The main enzymes involved in TNF-α and FasL shedding are ADAM-17, ADAM-10, MMP7, and MMP3. TRAIL shedding seems to involve cysteine protease activity, although no specific enzyme has been already described. Regulation of the shedding activity involves a family of proteins called TIMP