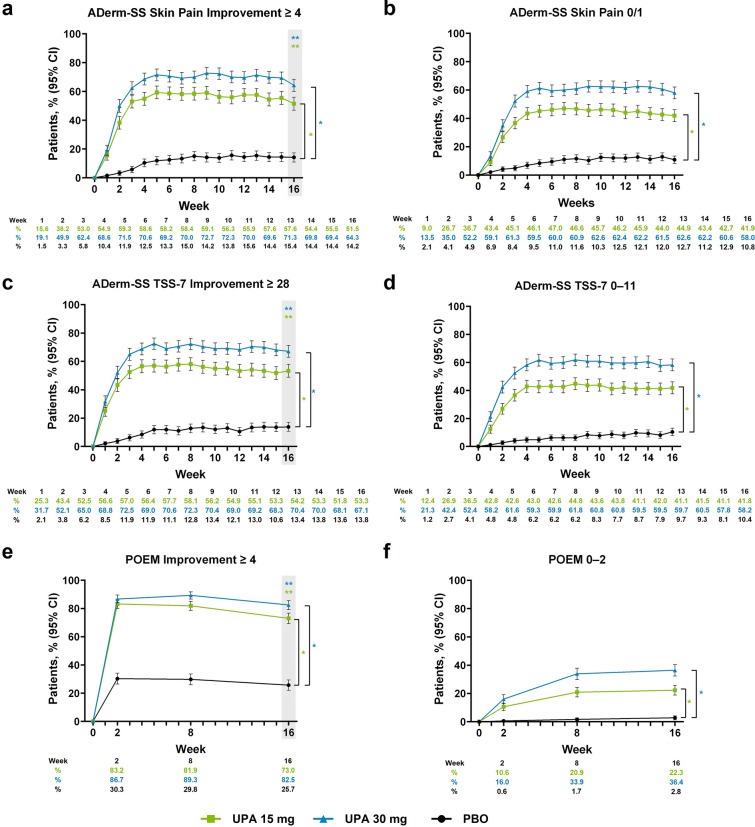
Fig. 2.
Patients enrolled in the Measure Up 1 and Measure Up 2 studies who achieved a ADerm-SS Skin Pain Improvement ≥ 4,a b ADerm-SS Skin Pain 0/1,b c ADerm-SS TSS-7 Improvement ≥ 28,c d ADerm-SS TSS-7 0–11,d e POEM Improvement ≥ 4,e and f POEM 0–2f during the double-blind period (NRI-C). Comparisons at time points in shaded boxes were multiplicity controlled. *Nominal P < 0.001 versus placebo for all time points. **Multiplicity adjusted P < 0.001 versus placebo. aAssessed in patients with ADerm-SS Skin Pain ≥ 4 at baseline. bAssessed in patients with ADerm-SS Skin Pain ≥ 2 at baseline. cAssessed in patients with ADerm-SS TSS-7 ≥ 28 at baseline. dAssessed in patients with ADerm-SS TSS-7 ≥ 12 at baseline. eAssessed in patients with POEM ≥ 4 at baseline. fAssessed in patients with POEM scores ≥ 3 at baseline. ADerm-SS Atopic Dermatitis Symptom Scale, CI confidence interval, NRI-C non-responder imputation incorporating multiple imputation for missing data due to COVID-19, PBO placebo, POEM Patient-Oriented Eczema Measure, TSS-7 7-item Total Symptom Score, UPA upadacitinib