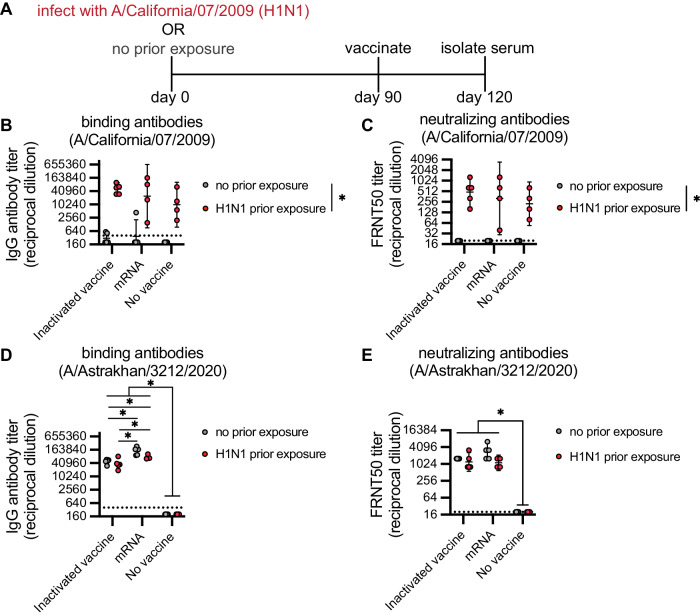
Fig. 5. Clade 2.3.4.4b H5 HA mRNA-LNP vaccine and inactivated H5 vaccine elicit robust antibody responses in mice previously exposed to H1N1 virus.
A Timeline for infections and vaccinations. Groups of 4-5 mice were uninfected or infected i.n. with 1000 TCID50 of A/California/07/2009 virus and then vaccinated i.m. 90 days later with either 1 µg of A/Astrakhan/3212/2020 HA mRNA-LNP (mRNA) or 50 HAU of an inactivated A/Astrakhan/3212/2020 vaccine (inactivated vaccine). Sera were isolated 30 days after vaccination and IgG antibody titers were measured using ELISA plates coated with HA from A/California/07/2009 (B) and A/Astrakhan/3212/2020 (D). 50% Foci reduction neutralization test (FRNT50) titers were determined using viruses expressing the HAs from A/California/07/2009 (C) and A/Astrakhan/3212/2020 (E). For the ‘no vaccine/no prior exposure’ group, we tested serum obtained from mice before initiating the experiment. All data are shown as geometric means ± 95% confidence intervals. Data were compared using two-way ANOVA with Tukey’s multiple comparisons test. Values were log-transformed before statistical analysis. Data are representative of 2 independent experiments. B Inactivated vaccine no prior exposure vs. no vaccine prior exposure *P = 0.0006; mRNA no prior exposure vs. no vaccine prior exposure *P = 0.0002; (C) Inactivated vaccine no prior exposure vs. no vaccine prior exposure *P = 0.0001; (D) Inactivated vaccine no prior exposure vs. no vaccine no prior exposure *P = 0.0444; all other comparisons *P < 0.0001. Red indicates mice with prior exposure. Source data are provided as a Source Data file.