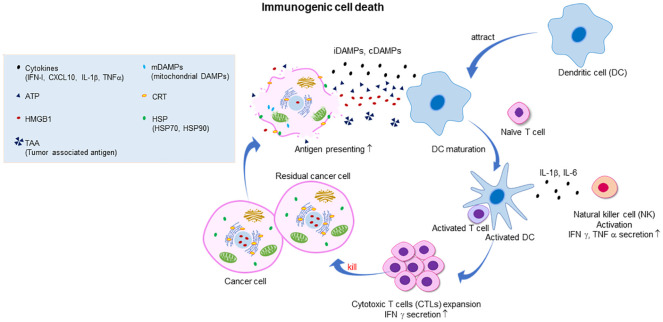
Figure 1.
Illustration of the mechanism of cancer immunogenic cell death (ICD) and the subsequent activation of the antitumor immune response. Dying cancer cells express various constitutive damage-associated molecular patterns (cDAMPs) and inducible DAMPs, including the release of high mobility group box 1 (HMGB1) from the nucleus, translocation and cell surface exposure of calreticulin (ecto-CRT) and heat shock proteins HSP70/90, and extracellular secretion of ATP, cytokines (such as type I IFN), chemokines (such as CXCL10), and nucleic acids. Exposure to DAMPs serves as a “find me” signal which recruits immature dendritic cells (DC) to tumor microenvironment (TME) and induces the maturation of DC. Ecto-CRT provides a pro-phagocytic “eat me” signal that promotes the phagocytosis of antigens by DC. In addition, HMGB1 and HSP70/90 assist in promoting the processing of phagocytic cargo by binding to toll-like receptors (TLRs), thereby escalating antigen engulfment, processing, and cross-presentation to CD8+ T cells to mediate robust tumor-specific immune response and protective immunological memory. Ecto-HSP70 and HSP90 also stimulate NK cell lysis. Primed CTLs elicit direct cytotoxic response and eradicate remaining tumor cells through the generation of IFN-γ, perforin-1 and granzyme B.