TABLE 1.
Optimization of the reaction conditions a .

| ||||||
|---|---|---|---|---|---|---|
| ||||||
| Entry | Base (eq.) | Cat | Solvent | t | Yield/% b | ee/% c |
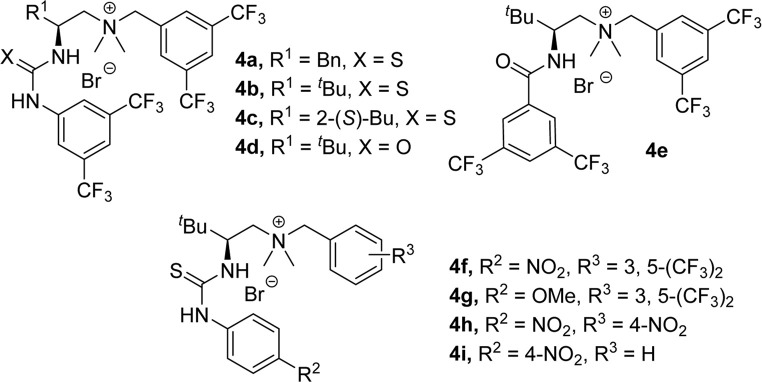
| 1 | K2CO3 (2.0) | 4a | Toluene | 12 h | 72 | 58 |
| 2 | K2CO3 (2.0) | 4b | Toluene | 12 h | 78 | 58 |
| 3 | K2CO3 (2.0) | 4c | Toluene | 12 h | 78 | 55 |
| 4 | K2CO3 (2.0) | 4d | Toluene | 5 h | 76 | 46 |
| 5 | K2CO3 (2.0) | 4e | Toluene | 2 day | 75 | −32 |
| 6 | K2CO3 (2.0) | 4f | Toluene | 12 h | 70 | 70 |
| 7 | K2CO3 (2.0) | 4g | Toluene | 1 day | 80 | 65 |
| 8 | K2CO3 (2.0) | 4h | Toluene | 3 day | 75 | 69 |
| 9 | K2CO3 (2.0) | 4i | Toluene | 2 day | 75 | 69 |
| 10 | Cs2CO3 (2.0) | 4f | Toluene | 5 h | 63 | 38 |
| 11 | K3PO4 (2.0) | 4f | Toluene | 18 h | 80 | 67 |
| 12 | NaOH (2.0) | 4f | Toluene | 12 h | 56 | 71 |
| 13 d | K2CO3 (2.0) | 4f | Toluene | 7 day | 77 | 76 |
| 14 | K2CO3 (1.0) | 4f | Toluene | 12 h | 85 | 75 |
| 15 | K2CO3 (0.1) | 4f | Toluene | 12 h | 80 | 74 |
| 16 | K2CO3 (0.1) | 4f | PhCF3 | 2.5 day | 65 | 68 |
| 17 | K2CO3 (0.1) | 4f | PhCl | 2 day | 88 | 71 |
| 18 | K2CO3 (0.1) | 4f | PhF | 2.5 day | 72 | 68 |
| 19 | K2CO3 (0.1) | 4f | THF | 2.5 day | 74 | 59 |
| 20 | K2CO3 (0.1) | 4f | 2-Me-THF | 2 day | 78 | 65 |
| 21 | K2CO3 (0.1) | 4f | Mesitylene | 1 day | 89 | 80 |
| 22 e | K2CO3 (0.1) | 4f | Mesitylene | 2.5 day | 88 | 80 |
| 23 | ‒ | 4f | Mesitylene | 1 day | ND | ‒ |
Unless otherwise noted, the reaction was performed with 0.10 mmol of 1a, 0.20 mmol of 2, catalyst 4 (5 mol%), and base in 1.0 mL solvent.
Isolated yield.
Determined by chiral HPLC analysis.
0°C.
4f (1 mol%) was used.
