Figure 4.
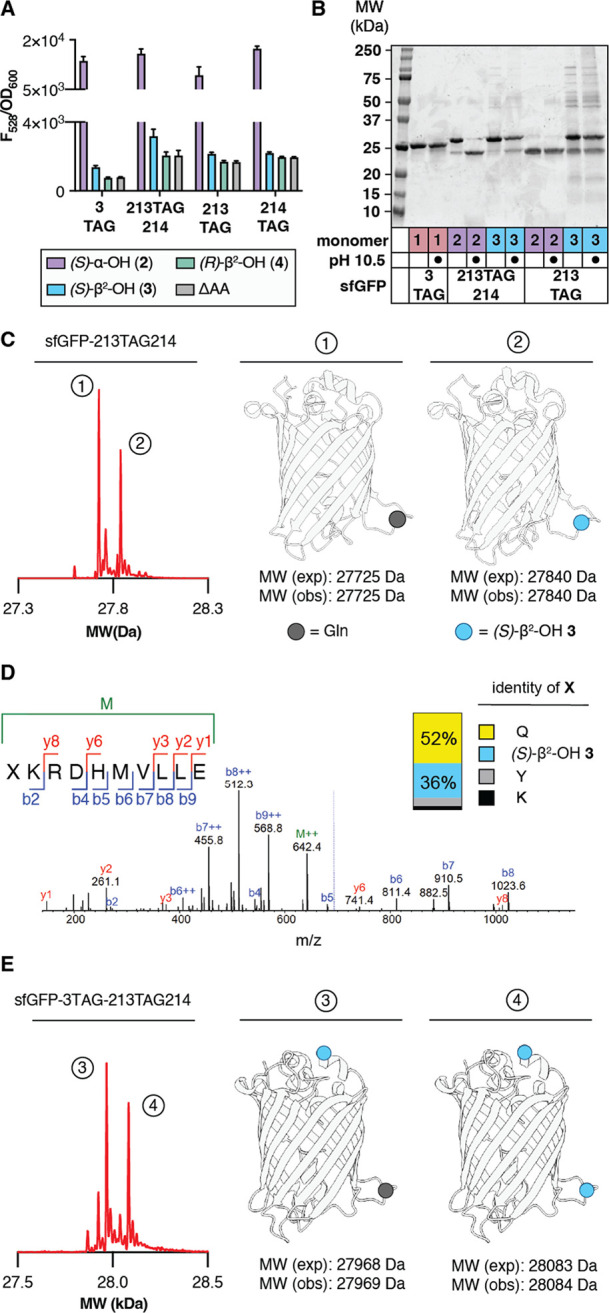
MaPylRS supports the in vivo synthesis of sfGFP containing one or two β2-hydroxy acid monomers. (A) Plot of F528/OD600 values of C321.ΔΑ.exp Ε. coli transformed with pMega-MaPylRS and the indicated sfGFP expression plasmid measured 24 h after induction with 1 mM IPTG as a function of monomer identity. (B) SDS-PAGE of the indicated isolated sfGFP variants after treatment with CAPS buffer (pH 10.5) or Milli-Q for 2 h at 37 °C. (C) Deconvoluted mass spectrum of sfGFP-213TAG214 expressed in the presence of (S)-β2-OH 3 reveals two products. One contains a single residue of 3, and the other contains a single residue of Gln. (D) MS/MS profile for peptide XKRDHMVLLE (where X is (S)-β2-OH 3) observed after GluC digestion of sfGFP-213TAG214 expressed in the presence of 0.1 mM (S)-β2-OH 3. Inset shows the fidelity of incorporation of (S)-β2-OH 3 relative to Gln, Tyr, or Lys. (E) Deconvoluted mass spectrum of sfGFP-3TAG-213TAG214 grown in defined media lacking Gln in the presence of 0.1 mM (S)-β2-OH 3. Two products are observed, whose masses correspond to (1) sfGFP with the addition of two copies of (S)-β2-OH 3 and (2) sfGFP with the addition of one copy of (S)-β2-OH 3 and a single Gln residue. GluC mapping data are found in Supporting Information Figure 9.
