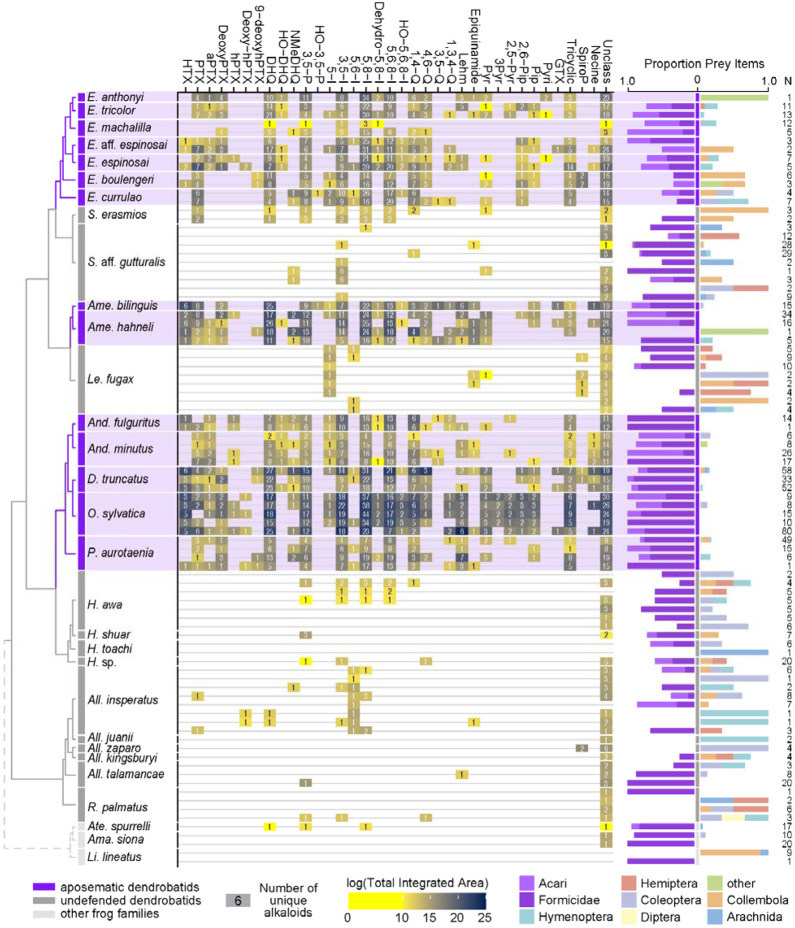
Figure 2.
From left to right: an ultrametric tree showing phylogenetic relationships inferred previously (Wan et al., 2023) among sampled species with the three defended poison frog clades highlighted in purple, the undefended clades in dark gray, and non-dendrobatids in light gray (Bufonidae: Amazophrynella siona and Atelopus aff. spurrelli; Leptodactylidae: Lithodytes lineatus). Tile color indicates the log of the total quantity of alkaloids in each class as measured by the sum of integrated areas of alkaloids of that class from GC-MS data per individual. The number in each tile indicates the number of alkaloids (including isomers) detected in each individual for each class. On the right are prey items recovered from the stomach of each individual, colored by arthropod group and scaled to 1 (total number of prey identified are shown under N). Note the large proportion of ants (Formicidae, dark purple) and mites (Acari, light purple) in many of the individuals compared to other prey types. See Table S1 for raw diet data and Table S4 for full alkaloid data. Poison-frog genera names are abbreviated as follows: All., Allobates; Ame., Ameerega; And., Andinobates; D., Dendrobates; E., Epipedobates; H., Hyloxalus; Le., Leucostethus; O., Oophaga; P., Phyllobates; R., Rheobates; S., Silverstoneia; Alkaloid class abbreviations are based on (Daly et al., 2009, 2005) and are as follows: HTX, histrionicotoxins; PTX, pumiliotoxins; PTXB, pumiliotoxin B; aPTX, allopumiliotoxins; DeoxyPTX, deoxypumiliotoxins; hPTX, homopumiliotoxins; deoxy-hPTX, deoxy-homopumiliotoxins; DHQ, decahydroquinolines; NMeDHQ, N-methyldecahydroquinolines; HO-DHQ, hydroxy-decahydroquinolines; 3,5-P, 3,5-disubstituted pyrrolizidines; HO-3,5-P, hydroxy-3,5-disubstituted pyrrolizidines; 5-I, 5-substituted indolizidines; 3,5-I, 3,5-disubstituted indolizidines; 5,6-I, 5,6-disubstituted indolizidines; 5,8-I, 5,8-disubstituted indolizidines; Dehydro-5,8-I, dehydro-5,8-indolizidines; 5,6,8-I, 5,6,8-trisubstituted indolizidines; HO-5,6,8-I, hydroxy-5,6,8-trisubstituted indolizidines; 1,4-Q, 1,4-disubstituted quinolizidines; 4,6-Q, 4,6-disubstituted quinolizidines; 3,5-Q, 3,5-disubstituted quinolizidines; 1,3,4-Q, 1,3,4-trisubstituted quinolizidines; Lehm, lehmizidines; Epiquinamide, epiquinamide; 2-Pyr, 2-substituted pyrrolidine; 3-Pyr, 3-substituted pyrrolidine; 2,5-Pyr, 2,5-disubstituted pyrrolidines; Pyr, pyrrolizidine of indeterminate substitution; 2,6-Pip, 2,6-disubstituted piperidines; Pip, other piperidines; Pyri, pyridines (including epibatidine); GTX, gephyrotoxins; Tricyclic, coccinelline-like tricyclics; SpiroP, spiropyrrolizidines; Necine, unspecified necine base; Unclass, unclassified alkaloids without known structures.