Abstract
Bordetella pertussis is the causative agent of pertussis or whooping cough. This bacterium is a human pathogen that under experimental conditions also infects selected rodents and primates. Here, we show for the first time that newborn piglets can be infected with B. pertussis when it is delivered intrapulmonarily. Infected piglets displayed fever and respiratory symptoms, such as nasal discharge, nonparoxysmal coughing, and breathing difficulties. Eventually, all infected animals developed severe bronchopneumonia, which in some cases was combined with a fibrinous pleuritits. Immunohistochemical staining revealed the presence of large numbers of B. pertussis cells within airways, adhering to the epithelial lining or phagocytosed by macrophages and neutrophils. Viable bacteria were reisolated from bronchoalveolar lavages and lung lesions for more than 10 days postinfection. The systemic presence of pertussis toxin was shown by hypoglycemia, lymphocytosis, and induction of a clustered pattern of CHO cells by serum and bronchoalveolar lavage samples. Thus, a large-animal model for pertussis was developed, which should complement existing rodent models for identifying the immune responses relevant to the design of new vaccines. In particular, this model should help researchers analyze the roles of both maternal and mucosal immunity in disease protection against pertussis and should ultimately assist in the design of new vaccines for early life protection.
Pertussis is a severe respiratory disease caused by the gram-negative bacterium Bordetella pertussis. Occasionally, infection with Bordetella parapertussis causes pertussis-like syndromes. Although this disease mainly afflicts young children worldwide, it is increasingly being recognized as a significant respiratory disease in adults (4, 19, 50). After an incubation phase, human pertussis begins with a catarrhal phase with symptoms typical of an upper respiratory infection. This is followed by attacks of paroxysmal coughing often associated with apnea and hypoxemia, although only a mild persistent cough may occur in older individuals. Complications such as pneumonia and bronchopneumonia may result, which often have a fatal consequence in young infants (51). Pertussis is more prevalent in developing countries, ranking fifth as a cause of global mortality due to infectious diseases (42). In recent years, however, interest in pertussis has emerged even in developed countries because of a resurgence of the disease (41, 50). Despite increasingly high vaccination rates among infants and children, the disease still afflicts up to 40 million children worldwide, with an annual death toll of 400,000 (50). In fact, the incidence of pertussis in some countries has been reported to have steadily increased since the 1980s (20, 41, 50).
B. pertussis, B. parapertussis, and Bordetella bronchiseptica are closely related bordetellae which can cause similar diseases in the upper respiratory tract. However, their host ranges vary significantly. While B. pertussis under natural conditions infects only humans, B. parapertussis strains can be classified into two groups, one infecting only humans and the other infecting only sheep (45). In contrast, B. bronchiseptica causes respiratory infections in a wide range of birds and mammals, including pigs (13), but only rarely infects humans (45, 49). Comparative genomic studies suggest that all bordetellae evolved from a common B. bronchiseptica-like ancestor. B. pertussis and B. parapertussis, however, adapted to more restricted host niches, which most likely was accomplished through a loss of functions that allowed these species to more effectively infect humans and sheep (5, 33). Under experimental conditions, however, both B. pertussis and B. parapertussis can infect other species. Thus, rodents, particularly mice, have been used to study pertussis, and in a few studies primates have been used. In contrast, the use of large-animal models, which might be more appropriate for human pertussis, has largely been ignored.
The pathogenesis of pertussis is exceptionally complex, and while many potential virulence factors have been described, the basis of protective immunity is still poorly understood. Natural infection results in potent induction of both humoral and cell-mediated immunity, which provides relatively long-lived protection against subsequent infection (29). Especially the induction of CD4+ T cells and the production of Th1-like cytokines are believed to be required for optimal disease protection (23, 29, 37). Vaccination with either whole-cell pertussis vaccines (wP) or acellular pertussis vaccines (aP) has significantly decreased the incidence of the disease worldwide (6). Interestingly, these vaccines induce different types of responses. Vaccination with wP induces an immune response with a Th-1 bias, whereas aP induces a response with a Th-2 bias (29). Current vaccination strategies are based on multiple parenteral immunizations during the first 6 months of life, but because of the failure to induce mucosal immunity, possible interference with maternal antibodies, and the Th2 bias in the newborn these vaccines still leave the infant less than 6 months old at highest risk of infection (50% of pneumonia cases [4]). Thus, improved vaccination strategies that can overcome these challenges are required, including the development of mucosal vaccines and maternal immunization as potential means of protection. A major obstacle for analyzing these new strategies, however, is the lack of suitable animal models. Here, we present a new disease model in newborn piglets, which resemble the human infant much more closely. The respiratory tract of newborn piglets has anatomical and immunological features that are more akin to those of humans than to those of rodents (26, 34). Furthermore, in pigs large amounts of immunoglobulin A (IgA) and IgG are transferred via colostrum and milk to the offspring and subsequently are transported to the mucosal surfaces via a highly similar secretion pathway. Thus, the pig represents a very valuable model for analyzing the role of maternal immunity in protection against pertussis. This model ultimately should lead to a better understanding of protective immunity and the development of more effective vaccines for the human infant.
MATERIALS AND METHODS
Bacterial culture.
Bacterial suspensions of strain Tohama I were stored at −70°C in Casamino Acids plus 10% glycerol. Organisms were initially grown on the surface of Bordet-Gengou (BG) (Becton, Dickinson and Company, United States) agar containing 15% (vol/vol) defibrinated sheep blood and 40 μg/ml of cephalexin (Sigma-Aldrich, United States) at 37°C for 48 h. After incubation, heavy inocula of bacteria were transferred to Stainer-Scholte (SS) medium and grown aerobically at 37°C for 48 h either as liquid cultures at 250 rpm in a Thermo Forma shaker or as BG agar plate cultures. Bacteria were harvested from BG agar plates by scraping them off and resuspending them in SS medium. Bacteria were collected by centrifugation at 2,500 × g for 10 min. The pellets were resuspended in phosphate-buffered saline without Mg2+ and Ca2+ (PBSA) (pH 7.2) and adjusted to the appropriate optical density at 600 nm using a spectrophotometer (Ultrospec 3000; Pharmacia Biotech, United Kingdom). The bacterial suspension (50% from liquid culture and 50% from BG agar plates) was kept on ice until it was used for challenge. The corresponding viable counts of the bacterial suspensions were determined by plating serial dilutions of the suspensions onto BG agar plates and incubating the plates at 37°C for 4 to 5 days.
Preparation of agar beads.
Microscopic agarose beads containing viable bacteria were prepared by mixing B. pertussis suspensions with molten 2% ion agar (Oxoid Ltd.) as previously described by Woods et al. (15, 48). Melted 2% ion agar in PBS (pH 7.0) was kept at 50°C, and an appropriate amount of a bacterial suspension in PBS was slowly added to obtain a final concentration of approximately 5 × 109 CFU B. pertussis/ml melted agar. Olive oil warmed at 50°C was vigorously stirred with a magnetic spin bar before a bacterial suspension was added to it. The oil-agar mixture was then rapidly cooled on ice. Beads were washed three times in PBS to remove excess oil. Control animals were inoculated with beads without bacteria.
In vitro growth inhibition assays.
Piglets, either 4 to 5 weeks old or newborn (colostrum deprived and colostrum fed), were euthanized, and bronchoalveolar lavages (BALs) were collected in SS medium. Alveolar macrophages and other cells were removed by centrifugation at 500 × g for 10 min before the assay was performed. BALs (290 μl) were cocultured in microtiter plates with 10 μl of bacteria (5 × 106 to 7 × 106 CFU) at 37°C for 6, 12, 24, and 48 h. At different times an incubated BAL was plated onto BG agar plates to evaluate the number of viable bacteria following incubation at 37°C for 4 to 5 days.
CHO cell assay.
The Chinese hamster ovary (CHO) cell assay was performed by the method of Hewlett et al. (17). CHO cells (ATCC CCl 61) grown to confluence were trypsinized and diluted in RPMI medium (Gibco, Invitrogen Corporation) supplemented with 10% fetal bovine serum and 1% antibiotic-antimycotic (Sigma Aldrich) to obtain a concentration of 3.0 × 104 cells per ml. A 250-μl portion of the suspension was added to each well of a flat-bottom microtiter plate. After incubation for 24 h at 37°C to allow attachment and stabilization of cells in the plates, 25 or 50 μl of BAL or serum of piglets was added to duplicate wells. Live bacteria were used as a positive control. PBSA, BAL, and serum from noninfected piglets were used as negative controls. After 24 h of incubation at 37°C in a CO2 incubator, the clustering effect of test samples was examined under an inverted microscope by different observers. The clustering was scored as no effect, some effect, or positive response.
Animals.
Pregnant Landrace sows were purchased from the Saskatoon Prairie Swine Centre, University of Saskatchewan. Sows were induced to farrow by intramuscular injection of prostaglandin (Planate; Schering, Quebec, Canada) at day 113 of gestation. All piglets were kept in the same room but in separated pens and were monitored very closely. Weaned piglets were purchased at an age of 25 days and after adjustment were infected at 30 days of age. Animals were seronegative and culture negative for B. bronchiseptica. All experiments were conducted in accordance with the ethical guidelines of the University of Saskatchewan and the Canadian Consul of Animal Care.
Intrapulmonary challenge of piglets.
A Micro-Renathane tube (size 0.95; Braintree Scientific Inc., United States) was sealed at the bottom end, and small holes were made for equal distribution of bacteria inside the lung. Piglets were anesthetized with isoflurane and intubated using a laryngoscope and an endotracheal tube (3 mm; Jorgensen Laboratories Inc., Loveland, CO). The Micro-Renathane tube was inserted through the endotracheal tube, and bacteria were delivered through the tube at a level of 1.5 ml/lung craniodorsal to the bronchial bifurcation. Control groups received either the same number of heat-inactivated bacteria (inactivated by heating at 56°C for 60 min) or PBS.
Postchallenge physiological measurements.
Animals were monitored daily, clinical symptoms such as fever, coughing, and respiratory problems were noted, and the pattern of weight gain and loss was recorded. In addition, piglets were bled every 2 days, and the total circulating leukocytes were counted (Cell Din 3500; Abbott Diagnostics, United States). Serum glucose levels were determined by the hexokinase method (Roche/Hitachi 704/911/912; Roche Diagnostics GmbH, Germany) at Prairie Diagnostic Services, University of Saskatchewan.
Postmortem investigation.
Piglets were euthanized by intraperitoneal injection of Euthanyl (sodium barbiturate; Bimeda-MTC, Ontario, Canada) at different times over a 21-day period postchallenge. The thoracic and abdominal cavities were opened and examined. The lungs were examined, and any lesions and abnormalities, such as pleuritis or local accumulations of blood and fluids in the thorax, were noted.
Quantification of bacteria from the lungs.
The lungs were removed following euthanasia, and the extent of pathological changes was determined macroscopically. The number of bacteria in the BAL and lesions was examined over a 21-day period. The BAL fluid was obtained by filling the lungs with 15 ml of SS medium and withdrawing as much fluid as possible (this procedure was performed once). To quantify B. pertussis in the BAL, fluid samples were centrifuged to remove debris and host cells. The supernatant and dilutions of the supernatant were plated onto BG agar plates in duplicate and incubated at 37°C for up to 1 week. To determine the number of bacteria within the tissues, lesions were excised, weighed, homogenized, and plated onto BG agar plates.
Histopathological and immunohistochemical staining of lung tissues.
Slices of the lung were fixed in 10% buffered formalin and processed using routine histological procedures (Thermo Shandon tissue processor; Thermo Shandon, Cheshire, England). The overnight process consisted of dehydration using graded ethanol, clearing using xylene, and infiltration using Paraplast wax. The tissues were then embedded in Paraplast wax. Five-micron-thick sections were cut, mounted on glass microscope slides, dried for a minimum of 1 h at 65°C, deparaffinized using xylene, and then rehydrated using graded ethanol and tap water. For conventional light microscopy the sections were stained in a routine manner using hematoxylin and eosin. Following dehydration and clearing, again using graded ethanol and xylene, the stained sections were sealed with coverslips and examined microscopically. Histopathological changes, including hemorrrhage, necrosis, bronchopneumonia, and pleuropneumonia, were evaluated on a scale indicating mild, moderate, and severe damage. Immunohistochemical staining was performed with formalin-fixed, paraffin-embedded sections by the indirect immunoperoxidase method with dextran polymer-conjugated secondary antibody labeled with peroxidase. In brief, after rehydration with PBS, the slides were blocked with avidin blocking solution, washed, incubated for 15 min with 0.3% H2O2 to eliminate endogenous peroxidase activity, incubated with mouse antipertactin monoclonal antibody, washed, and incubated with a secondary biotinylated goat anti-mouse conjugated antibody. The reaction was visualized using a 3,3′-diaminobenzidine solution and intensified with CuSO4, and the preparations were counterstained with Giemsa stain.
RESULTS
In the present study, five challenge experiments were performed to infect either 3-day-old piglets (newborn piglets) or 4- to 5-week-old piglets (Table 1). All animals were intrapulmonarily challenged with 5 × 109 to 7 × 109 CFU B. pertussis strain Tohama I. Newborn piglets were either colostrum fed or colostrum deprived (Table 1).
TABLE 1.
Summary of challenge experiments
| Expt | No. of animals | Age of animals (wk) | Challenge | Colostrum | No. positive/no. tested
|
|
|---|---|---|---|---|---|---|
| Respiratory symptoms | Pathological alterations | |||||
| I | 12 | Newborn | 5 × 109 CFU | + | 12/12 | 11/12 |
| 6 | Newborn | PBS | + | 0/6 | 0/6 | |
| II | 11 | Newborn | 5 × 109 CFU | − | 11/11 | 11/11 |
| 6 | Newborn | PBS | − | 2/6a | 2/6a | |
| III | 9 | Newborn | 7 × 109 CFU | + | 9/9 | 9/9 |
| 5 | Newborn | PBS | + | 0/5 | 0/5 | |
| 5 | Newborn | 5 × 109 CFU heat-inactivated bacteria | + | 0/5 | 0/5 | |
| IV | 12 | 4-5 | 5 × 109 CFU | + | 0/12 | 0/12 |
| 6 | 4-5 | PBS | + | 0/6 | 0/6 | |
| V | 10 | 4-5 | 5 × 109 CFU incorporated into agar beads | + | 0/10 | 0/10 |
| 6 | 4-5 | PBS | + | 0/6 | 0/6 | |
Bacteriological and histopathological examination revealed that respiratory infection in the piglets was not caused by B. pertussis.
Infection of 3-day-old piglets. (i) Inhibitory effect of BALs from uninfected animals against B. pertussis.
To analyze the bactericidal activity of BALs from uninfected animals, BALs obtained from newborn animals were cocultured with 1 × 106 CFU B. pertussis for 24 h (data not shown). No growth inhibition was observed with the BALs collected from either colostrum-fed or colostrum-deprived newborn piglets compared to the control wells (SS medium) after 6 h (data not shown) or 24 h of incubation at 37°C. Furthermore, uptake of colostrum and the presence of large amounts of nonspecific secreted-IgA and IgG antibodies in the BALs had no effect on the in vitro growth of B. pertussis (data not shown).
(ii) Susceptibility of newborn piglets to B. pertussis and physiological findings.
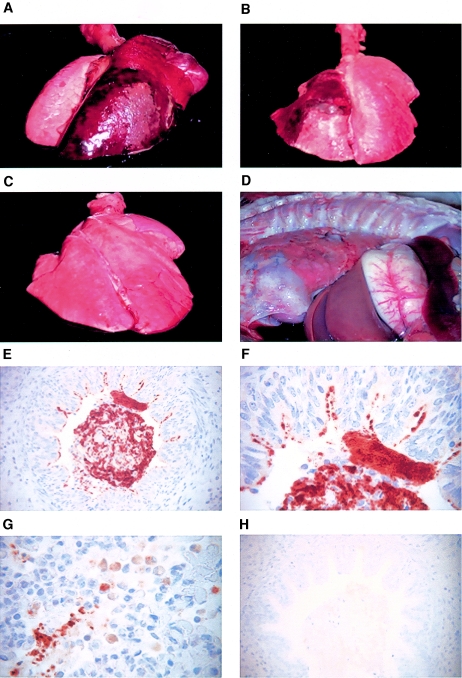
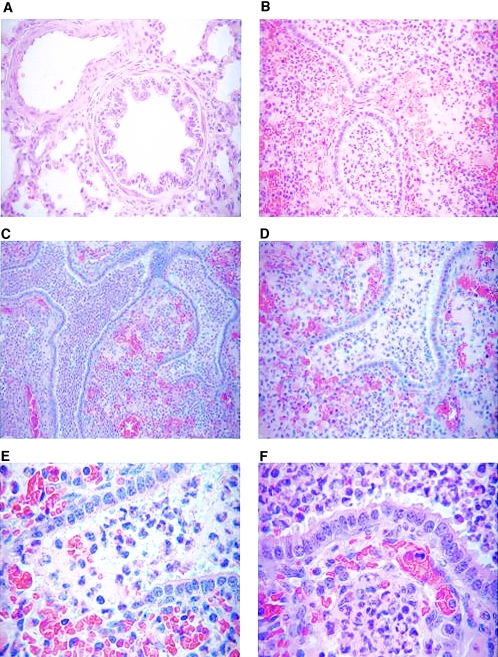
Newborn piglets were challenged with 5 × 109 to 7 × 109 CFU live bacteria. This number was based on previous studies in which 2 × 106 CFU or 2 × 108 CFU was used for challenge infection. None of these previous doses resulted in clinical symptoms, pathology, or isolation of bacteria from the lung (data not shown). The control piglets were divided into two groups; one group was challenged with 5 × 109 CFU heat-inactivated bacteria (56°C for 30 min), and the other group was treated with PBS. None of the control animals showed any clinical signs following inoculation. In contrast, all piglets infected with live bacteria displayed clinical symptoms, including fever and respiratory symptoms, such as nasal discharge, occasional nonparoxysmal cough, and breathing difficulties. Symptoms were observed as early as 2 days postchallenge and were found for up to 2 weeks following challenge. Furthermore, there was a pronounced retardation in weight gain in infected piglets during the period of observation (data not shown). The control animals had daily weight gains of 200 g to 400 g during the period of observation, whereas infected animals lost weight for 3 days and later gained weight at the same rate as the controls. Postmortem investigation and macroscopic examination indicated pathological alterations, such as hemorrhagic and necrotizing pneumonia (Fig. 1A and B) and fibrinous pleuritis (Fig. 1D) in the lungs of challenged piglets. In contrast, none of the control animals showed any pathological alterations in the lung (Fig. 1C). The extent of the lung lesions in infected piglets varied from 20% to 90% of the infected lobe depending on the duration of infection (Fig. 1A and B). Mortality was not seen before euthanasia, and bacteria were not recoverable from the blood. No differences in clinical signs or pathology were found between colostrum-deprived and colostrum-fed animals (data not shown).
FIG. 1.
Gross pathology and immunohistochemical staining of infected lung tissues. (A and B) Lungs from newborn piglets infected with 5 × 109 CFU B. pertussis at 2 days (A) and 4 days (B) after the challenge infection. (C) Lung from a newborn piglet treated with 5 × 109 CFU heat-killed B. pertussis at 2 days after treatment. (D) Severe fibrinous pleuritis in a newborn piglet infected with 5 × 109 CFU B. pertussis at 4 days after challenge. (E to H) Immunohistochemical staining of formalin-fixed paraffin-embedded lung sections from a newborn piglet infected with 5 × 109 CFU B. pertussis at 4 days after challenge. An antipertactin antibody (BB05; courtesy of A. Weiss) was used for detecting B. pertussis. Bacteria were found within the lumen of the airways (E) (magnification, ×400), adhering to the epithelial cells or phagocytosed by macrophages and neutrophils (F and G) (magnification, ×1,000). An isotype control (5 μg/ml) was included (H) (magnification, ×400).
(iii) Quantification of bacteria from the BALs and lung lesions of infected piglets.
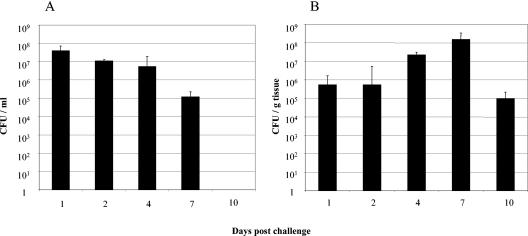
The lungs were removed, and the BAL fluid was obtained by filling the lungs with 15 ml of SS medium. The numbers of bacteria in the BALs (Fig. 2A) and lung lesions (Fig. 2B) were determined over a 21-day period. In BALs the number of isolated bacteria appeared to decrease during infection, and bacteria could still be isolated 7 days postinfection but not 10 days postinfection. In homogenized lung tissues, however, the number of bacteria appeared to increase during infection for up to 7 days postchallenge, and bacteria could still be detected in large numbers (>105 CFU/g tissue) even after 10 days. No bacteria could be isolated from nonaltered lung tissues (data not shown).
FIG. 2.
Isolation of B. pertussis from BALs and lung lesions of infected piglets. In each experiment two or three piglets infected with live bacteria were euthanized at different times postinfection. (A) BALs from all animals were collected, titrated, and plated onto BG agar plates to determine the numbers of viable bacteria in the BALs. Each bar indicates the mean for a minimum of eight animals, and the error bar indicates the standard error of the mean. (B) Macroscopically altered tissues were collected from the center of the lesions, weighed, and homogenized. To determine the number of CFU/g tissue, cleared supernatants were plated onto BG agar plates, and viable counts were determined. Each bar indicates the mean for a minimum of eight animals, and the error bar indicates the standard error of the mean.
(iv) Pulmonary inflammation following infection.
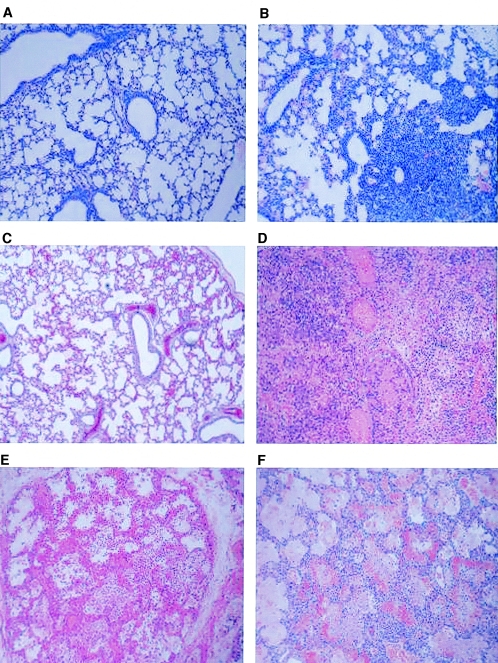
Histopathological examination of lung tissues from piglets infected with live B. pertussis revealed severe cellular infiltrations (neutrophils and macrophages) in the alveolar spaces, around the bronchioles, and in the walls of blood vessels (Fig. 3D and 4B to F). This pattern was associated with severe bronchiolar necrosis, vasculitis, alveolar hemorrhagic congestion and necrosis, fibrinous alveolar exudate, edema, and focal bronchiolar necrosis by day 4 (Fig. 4B to F). Numerous bacteria were also visible in the alveolar spaces. Severe subacute locally extensive hemorrhagic necrotizing pneumonia was noted, characterized by severe infiltration of neutrophils and macrophages in the alveolar spaces and in the bronchioles by day 7 (data not shown). In addition, there was evidence of moderate hyperplasia of the bronchiolar epithelium, moderate polyp formation (bronchiolitis obliterans) in some small bronchioles, and a mild thickening of alveolar walls and mild interlobular edema in some areas of lung parenchyma. By day 10, suppurative and histiocytic pleuropneumonia was characterized by extensive necrosis, and the surrounding parenchyma was extremely cellular and fibrotic. The airways within the fibrotic zone were narrowed and filled with neutrophils, but beyond this zone large activated macrophages were found (data not shown). By day 15, moderate, multifocal, peribronchiolar atelectasis with the presence of macrophages and scattered neutrophil infiltration in the alveolar spaces revealed mild chronic bronchointerstitial pneumonia (data not shown). For comparison, histological alterations found in lung biopsies taken from two children who died at 29 (Fig. 3E) or 37 (Fig. 3F) days of age after infection with B. pertussis were included. Additionally, lungs from a 21-day-old BALB/c mouse obtained 10 days after challenge with 5 × 108 CFU B. pertussis strain Tohama I (Fig. 3B) and a control mouse (Fig. 3A) were also included. Consistently in all three species, severe bronchopneumonia composed of mononuclear cells along with scattered alveolitis and parenchymal interstitial inflammation with lymphocytic and neutrophilic infiltration was found. Severe hemorrhagic congestion, edema, and focal necrosis were especially prominent in human and porcine cases and are commonly found in infected children (21, 27, 39).
FIG. 3.
Histopathology. (A and B) Photomicrographs of lung biopsies taken from a 21-day-old uninfected BALB/c mouse (A) and a BALB/c mouse infected with 5 × 108 CFU B. pertussis by aerosol (B) 10 days after challenge, obtained with a 10× objective plus digital zoom. (C and D) Photomicrographs of lung biopsies taken from newborn piglets 4 days after treatment with 5 × 109 CFU heat-inactivated B. pertussis (4× objective plus digital zoom) (C) or after challenge with 5 × 109 CFU B. pertussis (10× objective plus digital zoom) (D). (E and F) Photomicrographs of lung biopsies taken from two children who died at 29 (E) or 37 (F) days of age after infection with B. pertussis (10 × objective plus digital zoom).
FIG. 4.
Histopathology in pigs. (A) Photomicrograph of a lung biopsy taken from a 4-day-old noninfected piglet (40× objective plus digital zoom). (B to F) Photomicrographs of lung biopsies taken from 4-day-old piglets infected with 5 × 109 CFU B. pertussis. (B) 40× objective plus digital zoom. (C) 20× objective plus digital zoom. (D) 40× objective plus digital zoom. (E and F) 100× objective plus digital zoom.
(v) Association of B. pertussis with host inflammatory cells in the lung.
The localization of B. pertussis in the lungs of piglets was detected by histopathology and immunohistochemistry only in animals infected with live bacteria. B. pertussis colonies were found in the alveolar spaces and bronchioles. Bacteria were either free in the alveolar spaces, attached to bronchial and alveolar epithelial cells, or ingested by alveolar macrophages (Fig. 1E to G).
(vi) Pertussis toxin activity in infected piglets.
Pertussis toxin is known to elicit a variety of biological effects, such as hypoglycemia and lymphocytosis in vivo in human and rodents (17). Infected piglets exhibited a significant reduction in blood glucose levels on day 4 after infection (P < 0.006) (Table 2). The hypoglycemia continued throughout the study period compared with the control group (data not shown). In addition, infected piglets showed a significant circulating lymphocytosis compared with noninfected control animals (data not shown). The presence of pertussis toxin in the BAL and serum of piglets was assessed by the CHO cell assay. Typical clustered morphology patterns of CHO cells as a result of the presence of pertussis toxin in the samples were found in either BALs (days 1 to 10 postinfection) or serum (days 2 to 10 postinfection) from piglets infected with B. pertussis. No cytotoxicity was observed either in control wells incubated with PBS or in wells containing specimens from control piglets (data not shown). Thus, these results clearly indicate that the pertussis toxin was found in both serum and BAL for at least 10 days following infection.
TABLE 2.
Blood glucose levels
| Time (days) | Blood glucose concn (mmol/liter) (mean ± SD) |
|---|---|
| Noninfected controls | 8.68 ± 0.48 |
| 2 | 4.53 ± 1.50 |
| 4 | 6.81 ± 0.65 |
| 7 | 6.53 ± 0.73 |
| 11 | 6.78 ± 0.87 |
| 15 | 6.97 ± 1.27 |
| 19 | 7.60 ± 0.28 |
In summary, our results demonstrated that newborn piglets were susceptible to infection with B. pertussis. Since observations in mice and humans clearly indicate that newborn animals or infants are more susceptible to disease than adults, we investigated if older piglets are less susceptible to the disease. To this end, 4- to 5-week-old weaned piglets were intrapulmonarily infected with B. pertussis strain Tohama I.
Infection of 4- to 5-week-old piglets. (i) Inhibitory effect of BAL against B. pertussis.
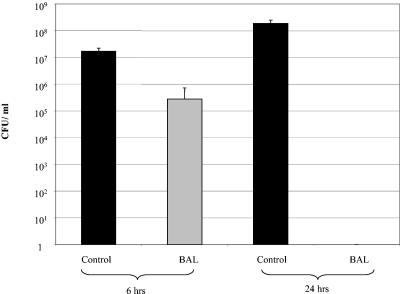
The bactericidal activity of BAL obtained from uninfected 4- to 5-week-old piglets was analyzed by coculturing 100 μl of BAL with 5 × 106 to 7 × 106 CFU B. pertussis in SS medium (Fig. 5). Compared to the control (medium), there was a significant time-dependent reduction in the number of viable bacteria in the BAL of 4- to 5-week-old piglets. Following 6 h of incubation there was about a 1.5-log reduction in the number of viable bacteria in the BAL of 4- to 5-week-old piglets compared to the control wells (bacteria plus medium). After 24 h, no viable bacteria were isolated after coculture with the BAL, demonstrating the complete neutralization of the bacteria by BALs from 4- to 5-week-old piglets against B. pertussis.
FIG. 5.
Inhibitory effect of BALs obtained from 4- to 5-week-old piglets. B. pertussis (5 × 106 to 7 × 106 CFU) was cocultured with BALs obtained from 4- to 5-week-old pigs (grey bar) or SS medium alone (black bars) for either 6 or 24 h. Supernatants were plated onto BG agar plates to determine the number of viable bacteria. Each bar indicates the mean for a minimum of six animals, and the error bar indicates the standard error of the mean.
(ii) Susceptibility of 4- to 5-week-old piglets to B. pertussis and physiological findings.
Intrapulmonary challenge of older piglets with 5 × 109 CFU did not result in any clinical symptoms for up to 3 weeks postchallenge. In addition, postmortem examination did not reveal any pathological alteration or lesions in the lungs of piglets for up to 21 days following challenge. Moreover, no viable bacteria were isolated from either the BALs or the lung tissues of challenged piglets (data not shown).
(iii) Infection of 4- to 5-week-old piglets with B. pertussis incorporated into agarose beads.
Since infection of older piglets was not achieved by intrapulmonary challenge with naked bacteria, we used the approach of Woods et al. (48) to induce infection by means of intrapulmonary instillation of agar beads containing viable bacteria. However, this also did not induce any clinical signs of infection or pathological changes in the lungs of piglets. Furthermore, we were not able to reisolate any viable bacteria from either the BALs or the lung tissues of piglets at different times postchallenge. Our results, therefore, indicate that older piglets were not susceptible to infection with B. pertussis.
DISCUSSION
Humans are considered the only natural host for B. pertussis, although rodent and primate animal models have been established to study the disease. Here, we demonstrate that newborn piglets can also be infected with 5 × 109 to 7 × 109 CFU B. pertussis strain Tohama I after intrapulmonary delivery. Given the size of the piglets, this dose is comparable to the 105 to 107 CFU routinely used for intranasal or aerosol infection of mice. Challenge doses of 108 CFU or less did not result in infection of piglets, and no bacteria were isolated from either BALs or the lung tissues of piglets. Following challenge with 5 × 109 CFU, however, for up to 10 days 107 to 108 CFU of viable B. pertussis was reisolated from BALs and lung tissues of infected piglets. Given the higher challenge dose, this number reflects findings for infected mice, from which usually between 106 and 107 CFU can be reisolated 10 to 12 days postinfection. However, viable bacteria could be reisolated from mice for up to 6 weeks postinfection (46), whereas in pigs viable bacteria in BALs were only found for up to 10 days postinfection. We believe that the difference is due to the different techniques used for isolation of the bacteria. Because of the size of the porcine lung, viable bacteria were reisolated by BAL and only to some extent by homogenization of pathologically altered lung tissues. In mice, however, the whole lung is routinely homogenized. It is much harder to wash adherent bacteria off the mucosa than to recover them by homogenization, and especially in inflamed or necrotic areas with a significant influx of inflammatory cells reisolation by BAL may be less effective. Consistent with this, viable bacteria were still found in homogenized lung lesions of piglets euthanized 12 days postinfection, whereas the corresponding BALs were negative.
Infected piglets exhibited a wide range of clinical symptoms, including nasal discharge, nonparoxysmal cough, breathing difficulties, and weight retardation. Similar symptoms can be found in human adults, including mild upper respiratory tract symptoms, cough, and mild fever. In infants, however, the symptoms are more severe and are often combined with the typical paroxysmal cough that results in apnea, hypoxemia, and asphyxia. Interestingly, the paroxysmal cough is observed only in human infants and rats and not in mice or piglets (48). Mortality in general is observed only in young infants with complications and in experimentally infected neonatal mice. In the present pig model none of the animals died as a direct result of the infection, and even at doses of 5 × 1010 CFU mortality was not observed (data not shown). Challenge doses higher than 5 × 1010 CFU have not been tested yet. Lymphocytosis and hypoglycemia, both of which are manifestations of pertussis toxin (18, 48), were also observed in infected piglets. Thus, our data indicate that B. pertussis effectively replicated in the porcine lung and released pertussis toxin systemically.
The histopathological alterations following B. pertussis infection in humans include marked lesions of the ciliated epithelium in the trachea and larger airways (Fig. 3E and F). Typically, after B. pertussis has bound to ciliated cells, damage to the epithelium is caused by secretion of inducible nitric oxide synthase by secretory cells, which is induced by the bacterial tracheal cytotoxin and endotoxin (10, 11).
In the present study B. pertussis bound to ciliated porcine cells was not analyzed. In humans, bronchopneumonia composed of mononuclear cells along with scattered alveolitis is either directly caused by the bacteria (27, 39) or due to secondary infections. Additionally, parenchymal interstitial inflammation with lymphocyte infiltration into alveolar septa can be commonly found (21). In infected piglets a dramatic influx of macrophages and neutrophils into airways and interstitial tissues was observed, resulting in severe bronchopneumonia with necrosis and in some cases fibrinous pleuritis. However, neither clinical nor pathological alterations were observed when pigs were challenged with heat-killed inactivated bacteria. This strongly supports the hypothesis that B. pertussis was the primary cause of bronchopneumonia in these piglets and is consistent with the observed association of B. pertussis in bronchopneumonia in humans, as shown in autopsy studies (27, 44, 48). For comparison, lung biopsies taken from two human infants who died at a very early age were included to demonstrate the similarities in pathological alterations between humans and pigs. In summary, we developed a new animal model which should help researchers further analyze the pathogenesis of the disease, as well as the host's immune response against B. pertussis.
The current vaccination strategies against pertussis consist of two or three immunizations within the first 6 months of life. However, neonates too young to have received a complete set of immunizations are still at the highest risk of pertussis infection (47). Thus, early vaccination at birth and maternal immunization strategies may be needed to increase the level of protection in this age group (7, 8, 12, 14). We intend to use the present model to validate and optimize maternal immunization as a strategy for neonatal protection. Evidence that maternal immunity plays an important role in protection against pertussis comes from early studies of women which demonstrated that immunization of pregnant women enhanced the neutralizing activity of a newborn's serum against pertussis (24). Also, Oda et al. (31) reported that purified colostral antibodies from mothers could protect newborn mice against aerosol infection. Furthermore, immunization of pregnant mice showed that transcolostral immunity provided better protection than transplacental immunity (30, 31).
Maternal immunization and the increase in passive immunity in the newborn, however, also have a downside in that maternal antibodies (matAbs) can interfere with active immunization of the newborn (2, 38, 43). Recent evidence suggests that it is primarily the ratio of matAbs to vaccine antigen that determines the outcome of the vaccination in the newborn (38). For pertussis, it is thought that matAbs have an inverse effect on vaccination with wP but not vaccination with aP (9, 16). Our model should enable us to investigate this issue in greater detail. Furthermore, we will also analyze the influence of age on immunization, as it has been shown that human neonates produce vigorous antibody responses after vaccination at birth and display higher antibody titers at an age of 5 months following a booster immunization (3). Another way of circumventing the possible interference between matAbs and active vaccination may be found in delivering the vaccine via the mucosal surfaces (28, 32). Local immunity in the upper respiratory tract is crucial for preventing colonization by B. pertussis. After natural infection, high titers of anti-filamentons hemagglutinin IgA antibodies were found in nasal secretions of convalescent patients (25), indicating the importance of these antibodies for disease protection. Immunization with either formalin-fixed bacteria, purified filamentons hemagglutinin, or detoxified pertussis toxin resulted in protection against challenge infection with B. pertussis (22, 23, 36, 40). However, to more thoroughly investigate mucosal effector mechanisms, animal models that enable us to investigate various immune parameters are required. Although the murine model is the most commonly used model, it has its limitations in its similarity to humans, the number of cells that can be recovered from the individual mucosal compartments (i.e., antigen-specific cells isolated from mucosa and lymph nodes), and the limited size of the samples, such as saliva, colostrum, milk, and BAL. Since all mucosal compartments are accessible in piglets, the present model should help us understand the importance of mucosal immunity in disease protection and finally lead to the development of more effective vaccines.
The present study showed that in contrast to newborn piglets, older piglets were resistant to infection by B. pertussis. Their response was both rapid and independent of previous exposure, highlighting the effectiveness of innate immunity at the respiratory mucosal surfaces. The susceptibility of newborn piglets suggested that innate immunity is regulated developmentally. We hypothesize that protection in older piglets is associated with the presence of innate immune components, such as antimicrobial peptides in the lung (1, 35). Preliminary studies suggest that antimicrobial peptides present in mucosal secretions of older piglets are crucial to the resistance against B. pertussis.
In summary, we developed a new porcine model of pertussis which should enable us to investigate the role of mucosal and maternally derived immunity in pathogenesis and disease protection against pertussis. Ultimately, this should assist us in the development of more effective mucosal vaccines against pertussis, which hopefully will provide protection at a very early age.
Acknowledgments
We thank the VIDO animal care staff, especially K. Mirakhur and A. Giesbrecht, for their invaluable help with housing, challenging, and monitoring the animals. We greatly appreciate the help of K. West at Prairie Diagnostic Services, University of Saskatchewan, for developing the immunohistochemical staining procedure, and we are thankful for antibody BB05 provided by A. Weiss. We also thank C. Wojnarowicz at Prairie Diagnostic Services, University of Saskatchewan, for help with necropsies and J. Wright at Dalhousie University and the IWK Health Centre for histopathological analysis of lung biopsies taken from children who died at an early age. We thank J. Wright at Dalhousie University and the IWK Health Centre for providing the micrographs in Fig. 3A, B, E, and F.
Funding was provided by the Canadian Institutes of Health Research, the Canadian Bacterial Diseases Network, and the Saskatchewan Health Research Foundation (postdoctoral fellowship to S.E.).
Editor: A. D. O'Brien
REFERENCES
- 1.Bals, R., and P. S. Heimstra. 2004. Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. Eur. Respir. J. 23:327-333. [DOI] [PubMed] [Google Scholar]
- 2.Baraff, L. J., R. D. Leake, D. G. Burstyn, T. Payne, C. L. Cody, C. R. Manclark, S. J. W. St. Geme, Jr. 1984. Immunologic response to early and routine DTP immunization in infants. Pediatrics 73:37-42. [PubMed] [Google Scholar]
- 3.Belloni, C., A. De Silvestri, C. Tinelli, M. A. Avanzini, M. Marconi, F. Strano, G. Rondini, and G. Chirico. 2003. Immunogenicity of a three component acellular pertussis vaccine administered at birth. Pediatrics 111:1042-1045. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 1995. Pertussis—United States, Jan. 1992 to June 1995. Morb. Mortal. Wkly. Rep. 44:525-529. [PubMed] [Google Scholar]
- 5.Cummings, C. A., M. M. Brinig, P. W. Lepp, S. van de Pas, and D. A. Relman. 2004. Bordetella species are distinguished by patterns of substantial gene loss and host adaptation. J. Bacteriol. 186:1484-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwards, K. M., M. D. Decker, and E. A. Mortimer, Jr. 1999. Pertussis vaccine, p. 293-344. In S. A. Plotkin and W. A. Orenstein (ed.), Vaccines, 3rd ed. W. B. Saunders, Philadelphia, Pa.
- 7.Edwards, K. M. 2003. Pertussis: an important target for maternal immunization. Vaccine 21:3483-3486. [DOI] [PubMed] [Google Scholar]
- 8.Edwards, K. M., and N. Halasa. 2003. Are pertussis fatalities in infants on the rise? What can be done to prevent them? J. Pediatr. 143:552-553. [DOI] [PubMed] [Google Scholar]
- 9.Englund, J. A., E. L. Anderson, G. F. Reed, M. D. Decker, K. M. Edwards, M. E. Pinchichero, M. C. Steinhoff, M. B. Rennels, A. Defroest, and B. D. Meade. 1995. The effect of maternal antibody on the serologic response and the incidence of adverse reaction after primary immunization with acellular and whole-cell pertussis vaccine combined with diphtheria and tetanus toxoids. Pediatrics 96:580-584. [PubMed] [Google Scholar]
- 10.Flak, T. K., and W. E. Goldman. 1996. Autotoxicity of nitric oxide in airway disease. Am. J. Respir. Crit. Care Med. 154:202-206. [DOI] [PubMed] [Google Scholar]
- 11.Flak, T. K., and W. E. Goldman. 1999. Signaling and cellular specificity of airway nitric oxide production in pertussis. Cell. Microbiol. 1:51-60. [DOI] [PubMed] [Google Scholar]
- 12.Gall, S. A. 2003. Maternal immunization. Obstet. Gynecol. Clin. N. Am. 30:623-636. [DOI] [PubMed] [Google Scholar]
- 13.Giles, C. J. 1992. Bordetellosis, p. 436-445. In A. D. Leman, B. E. Straw, W. L. Mengeling, S. D'Allaire, and D. J. Taylor (ed.), Diseases of swine. Iowa State University Press, Ames.
- 14.Glenzen, W. P., and M. Alpers. 1999. Maternal immunization. Clin. Infect. Dis. 28:219-224. [DOI] [PubMed] [Google Scholar]
- 15.Hall, E., R. Parton, and A. C. Wardlaw. 1997. Differences in coughing and other responses to intrabronchial infection with Bordetella pertussis among strains of rats. Infect. Immun. 65:4711-4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Healy, C. M., F. M. Munoz, M. A. Rench, N. B. Halasa, K. M. Edwards, and C. J. Baker. 2004. Prevalence of pertussis antibodies in maternal delivery, cord, and infant serum. J. Infect. Dis. 190:335-340. [DOI] [PubMed] [Google Scholar]
- 17.Hewlett, E. L., K. T. Sauer, G. A. Myers, J. L. Cowell, and R. L. Guerrant. 1983. Induction of a novel morphological response in Chinese hamster ovary cells by pertussis toxin. Infect. Immun. 40:1198-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodge, G., S. Hodge, C. Markus, A. Lawrence, and P. Han. 2003. A marked decrease in L-selectin expression by leucocytes in infants with Bordetella pertussis infection: leucocytosis explained? Respirology 8:157-162. [DOI] [PubMed] [Google Scholar]
- 19.Ivanoff, B., and S. E. Robertson. 1997. Pertussis: a worldwide problem. Dev. Biol. Stand. 89:3-13. [PubMed] [Google Scholar]
- 20.James, D. C. 2003. The science and fiction of the resurgence of pertussis. Pediatrics 112:405-406. [DOI] [PubMed] [Google Scholar]
- 21.Khelef, N., C. M. Bachelet, B. B. Vargaftig, and N. Guiso. 1994. Characterization of murine lung inflammation after infection with parental Bordetella pertussis and mutants deficient in adhesins or toxins. Infect. Immun. 62:2893-2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee, H. Y., A. Andalibi, P. Webster, S. K. Moon, K. Teufert, S. H. Kang, J. D. Li, M. Nagura, T. Ganz, and D. J. Lim. 2004. Antimicrobial activity of innate immune molecules against Streptococcus, Moraxella catarrhalis and nontypeable Haemophilus influenzae. BMC Infect. Dis. 4:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leef, M., K. L. Elkins, J. Barbic, and R. D. Shahin. 2000. Protective immunity to Bordetella pertussis requires both B cells and CD4+ T cells for key functions other than specific antibody production. J. Exp. Med. 191:1841-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lichty, J. A., Jr., B. Salvin, and W. L. Bradford. 1938. An attempt to increase resistance to pertussis in newborn infants by immunizing their mothers during pregnancy. J. Clin. Investig. 17:613-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Long, S. S., C. J. Welkon, and J. C. Clark. 1990. Widespread silent transmission of pertussis in families: antibody correlates of infection and symptomatology. J. Infect. Dis. 161:480-486. [DOI] [PubMed] [Google Scholar]
- 26.MacKenzie, D. A., D. A. Hullett, and H. W. Sollinger. 2003. Xenogeneic transplantation of porcine islets: an overview. Transplantation 76:887-891. [DOI] [PubMed] [Google Scholar]
- 27.Mallory, F. B., and A. A. Horner. 1912. Pertussis: the histological lesion in the respiratory tract. J. Med. Res. 27:115-123. [PMC free article] [PubMed] [Google Scholar]
- 28.McKenzie, B. S., J. L. Brady, and A. M. Lew. 2004. Mucosal immunity: overcoming the barrier for induction of proximal responses. Immunol. Res. 30:35-72. [DOI] [PubMed] [Google Scholar]
- 29.Mills, K. H. G. 2001. Immunity to B. pertussis. Microbes Infect. 3:655-677. [DOI] [PubMed] [Google Scholar]
- 30.Oda, M., K. Izumiya, Y. Sato, and M. Hirayama. 1983. Transplacental and transcolostral immunity to pertussis in a mouse model using acellular pertussis vaccine. J. Infect. Dis. 148:138-145. [DOI] [PubMed] [Google Scholar]
- 31.Oda, M., J. L. Cowell, D. G. Burstyn, S. Thaib, and C. Manclark. 1985. Antibodies to Bordetella pertussis in human colostrum and their protective activity against aerosol infection in mice. Infect. Immun. 47:441-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogra, P. L., H. Faden, and R. C. Welliver. 2001. Vaccination strategies for mucosal immune responses. Clin. Microbiol. Rev. 14:430-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parkhill, J., M. Sebaihia, A. Preston, L. D. Murphy, N. Thomson, D. E. Harris, M. T. Holden, C. M. Churcher, S. D. Bentley, K. L. Mungall, A. M. Cerdeno-Tarraga, L. Temple, K. James, B. Harris, M. A. Quail, M Achtman, R. Atkin, S. Baker, D. Basham, N. Bason, I. Cherevach, T. Chillingworth, M. Collins, A. Cronin, P. Davis, J. Doggett, T. Feltwell, A. Goble, N. Hamlin, H. Hauser, S. Holroyd, K. Jagels, S. Leather, S. Moule, H. Norberczak, S. O'Neil, D. Ormond, C. Price, E. Rabbinowitsch, S. Rutter, M. Sanders, D. Saunders, K. Seeger, S. Sharp, M. Simmonds, J. Skelton, R. Squares, S. Squares, K. Stevens, L. Unwin, S. Whitehead, B. G. Barrell, and D. J. Maskell. 2003. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Nat. Genet. 35:32-40. [DOI] [PubMed] [Google Scholar]
- 34.Rothkotter, H. J., E. Sowa, and R. Pabst. 2002. The pig as a model of developmental immunology. Hum. Exp. Toxicol. 21:533-536. [DOI] [PubMed] [Google Scholar]
- 35.Schnapp, D., and A. Harris. 1998. Antibacterial peptides in bronchoalveolar lavage fluid. Am. J. Respir. Cell Mol. Biol. 19:352-356. [DOI] [PubMed] [Google Scholar]
- 36.Shahin, R. D., M. J. Brennan, Z. M. Li, B. D. Meade, and C. R. Manclark. 1990. Characterization of the protective capacity and immunogenicity of the 69-kD outer membrane protein of Bordetella pertussis. J. Exp. Med. 171:63-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shumilla, J. A., V. Lacaille, T. M Hornell, J. Huang, S. Narasimhan, D. A. Relman, and E. D. Mellins. 2004. Bordetella pertussis infection of primary human monocytes alters HLA-DR expression. Infect. Immun. 72:1450-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siegrist, C. A. 2003. Mechanisms by which maternal antibodies influence infant vaccine responses: review of hypotheses and definition of main determinants. Vaccine 21:3406-3412. [DOI] [PubMed] [Google Scholar]
- 39.Smith, L. W. 1927. The pathologic anatomy of pertussis. Arch. Pathol. Lab. Med. 4:732-742. [Google Scholar]
- 40.Stevenson, A., and M. Roberts. 2003. Use of Bordetella bronchiseptica and Bordetella pertussis as live vaccines and vectors for heterologous antigens. FEMS Immunol. Med. Microbiol. 37:121-128. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka, M., C. Vitek, F. B. Pascual, K. M. Bisgard, J. S. Tate, and T. V. Murphy. 2003. Trends in pertussis among infants in the United States, 1980-1999. JAMA 290:2968-2975. [DOI] [PubMed] [Google Scholar]
- 42.Ulmer, J. B., and M. A. Liu. 2002. Ethical issues for vaccines and immunization. Nat. Rev. Immunol. 2:291-296. [DOI] [PubMed] [Google Scholar]
- 43.Van Savage, J., M. D. Decker, K. M. Edwards, S. H. Sell, and D. T. Karzon. 1990. Natural history of pertussis antibody in the infant and effect on vaccine response. J. Infect. Dis. 161:487-492. [DOI] [PubMed] [Google Scholar]
- 44.Vegelin, A. L., A. J. van Vught, T. F. Wolfs, J. I. Kimpen, and S. P. Geelen. 1998. Pertussis in young infants. Ned. Tijdschr. Geneeskd. 142:2657-2660. [PubMed] [Google Scholar]
- 45.Vinogradov, E., M. S. Peppler, and M. B. Perry. 2000. The structure of the nonreducing terminal groups in the O-specific polysaccharides from two strains of B. bronchiseptica. Eur. J. Biochem. 267:7230-7236. [DOI] [PubMed] [Google Scholar]
- 46.Watanabe, M., and M. Nagai. 2003. Role of systemic and mucosal immune responses in reciprocal protection against Bordetella pertussis and Bordetella parapertussis in a murine model of respiratory infection. Infect. Immun. 71:733-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wirsing von Konig, C. H., S. Halperin, M. Riffelmann, and N. Guiso. 2002. Pertussis of adults and infants. Lancet Infect. Dis. 2:744-750. [DOI] [PubMed] [Google Scholar]
- 48.Woods, D. E., R. Franklin, S. J. Cryz, M. Ganss, M. Peppler, and C. Ewanowich. 1989. Development of a rat model for respiratory infection with Bordetella pertussis. Infect. Immun. 57:1018-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woolfrey, B. F., and J. A. Moody. 1991. Human infections associated with Bordetella bronchiseptica. Clin. Microbiol. Rev. 4:243-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.World Health Organization. 1999. Pertussis vaccines: W.H.O. position paper. Wkly. Epidemiol. Rec. 74:137-144.10355354 [Google Scholar]
- 51.Wortis, N., P. M. Strebel, M., Wharton, B. Bardeneir, and I. R. Hardy. 1996. Pertussis deaths: report of 23 cases in the united states, 1992 and 1993. Padiatrics 97:607-612. [PubMed] [Google Scholar]