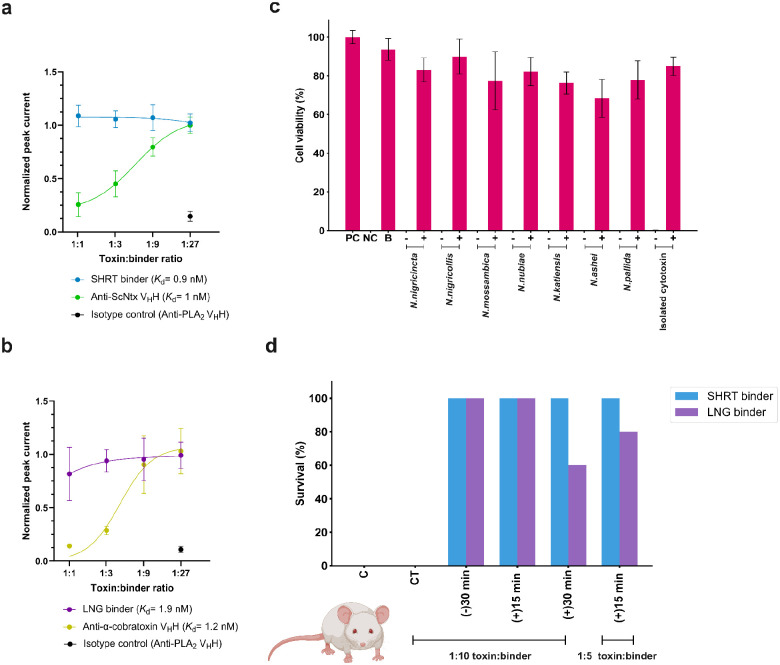
Figure 4. In vitro and in vivo efficacy of designed proteins against snake venom toxins.
(a) Concentration-response curves comparing SHRT binder and anti-ScNtx VHH efficacy in preventing nAChR blocking by 1 IC80 of ScNtx. Data represent the toxin’s inhibition of ACh response, normalized to full ACh response, averaged within each group (n=16). (b) Concentration-response curves comparing the efficacy of LNG binder and anti-α-cobratoxin VHH in preventing nAChR blocking by 1 IC80 of α-cobratoxin. (c) Neutralization of the cytolytic effects of whole venoms from seven different Naja (N.) species and isolated cytotoxin by the CYTX binder. 2 IC50 of the whole venoms or toxin were pre-incubated with CYTX at a 1:5 molar ratio (toxin:binder). Keratinocyte media was used as a positive control (PC). Triton X-100 was used as a negative control (NC). CYTX binder (B) was used as a positive control. (−) denotes 2 IC50 of the whole venoms without binder, and (+) denotes venoms incubated with binder. Experiments were performed in triplicates, and results are expressed as mean ± SD. (d) Mice survival following lethal neurotoxin challenge (n=5). 3 LD50s of ScNtx or α-cobratoxin were preincubated for 30 minutes (−30 min) with the corresponding protein binders at 1:10 ratios and then administered IP into groups of five mice. Toxins administered IP following IP administration of binders at 1:10 or 1:5 molar ratios (toxin:binder) either after 15 (+15 min) or 30 minutes (+30 min) post-toxin injection. Controls included mice receiving toxins alone (C). Specificity was assessed via cross-treatment (CT) experiments, where non-target binders were preincubated with 3 LD50s of ScNtx or α-cobratoxin and administered IP. Signs of toxicity were observed, and deaths were recorded for a period of 24 hours. (d) was created with BioRender.com.