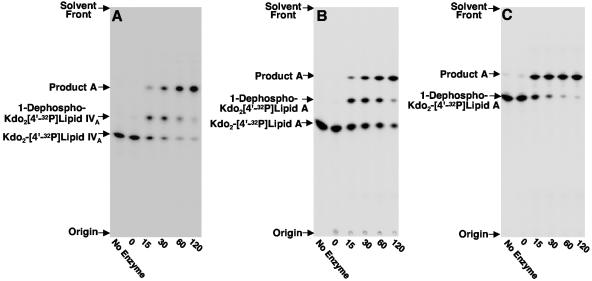
FIG. 2.
Enzymatic modification of Kdo2-[4′-32P]lipid IVA (panel A), Kdo2-[4′-32P]lipid A (panel B), and 1-dephospho-Kdo2-[4′-32P]lipid A (panel C) using H. pylori membranes. Membranes from H. pylori strain J99 were assayed for enzymatic activities that modify the Kdo2-lipid A domain of LPS as described in Materials and Methods. The protein concentration was 1.0 mg/ml, and assays were carried out for the indicated times at 30°C. Reaction mixtures contained either 5 μM Kdo2-[4′-32P]lipid IVA (panel A), 5 μM Kdo2-[4′-32P]lipid A (panel B), or 5 μM 1-dephosphorylated Kdo2-[4′-32P]lipid A (panel C). Reaction products were separated by TLC and detected with PhosphorImager analysis. The previously characterized 1-dephosphorylated reaction products catalyzed by Hp0021 are indicated, and the unknown reaction product was designated product A. Similar results were obtained when membranes from H. pylori strain 26695 were used as the enzyme source (data not shown).