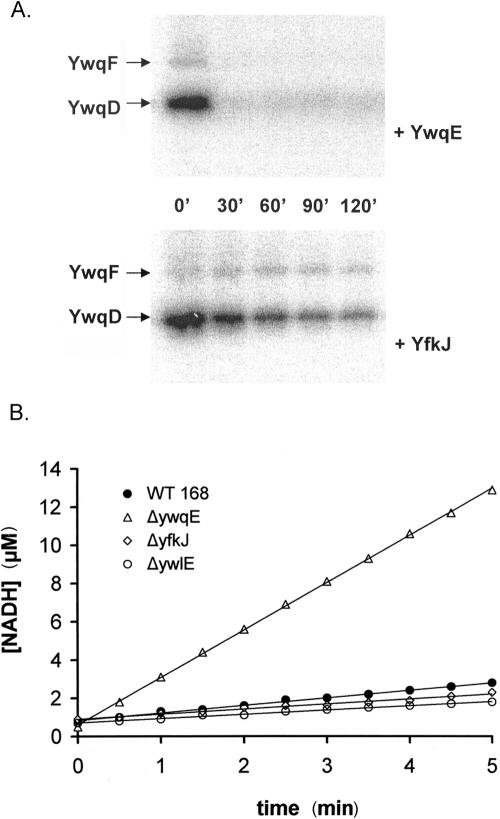
FIG. 4.
(A) In vitro dephosphorylation of phospho-YwqD and phospho-YwqF by YwqE and YfkJ. Autoradiograms of SDS-PAGE gels with 1 μM autophosphorylated YwqD and 10 μM phosphorylated UDP-glucose dehydrogenase YwqF. 32P-proteins were incubated with either 1 μM YwqE (upper panel) or 1 μM GST-YfkJ (lower panel), in the presence of 5 mM MnCl2, for the indicated time at 37°C. (B) In vivo analysis of redundancy between YwqE and B. subtilis LMPTPs. The graph shows UDP-glucose dehydrogenase activities of B. subtilis dialyzed protein extracts. The amount of extract used in the assay was normalized with respect to total protein concentration. The different extracts used were prepared from wild-type strain 168 (filled circles), and the ΔywqE (open triangles), ΔyfkJIH (open diamonds), and ΔywlE (open circles) strains. The concentrations of produced NADH were calculated from the absorbance variations at 340 nm.