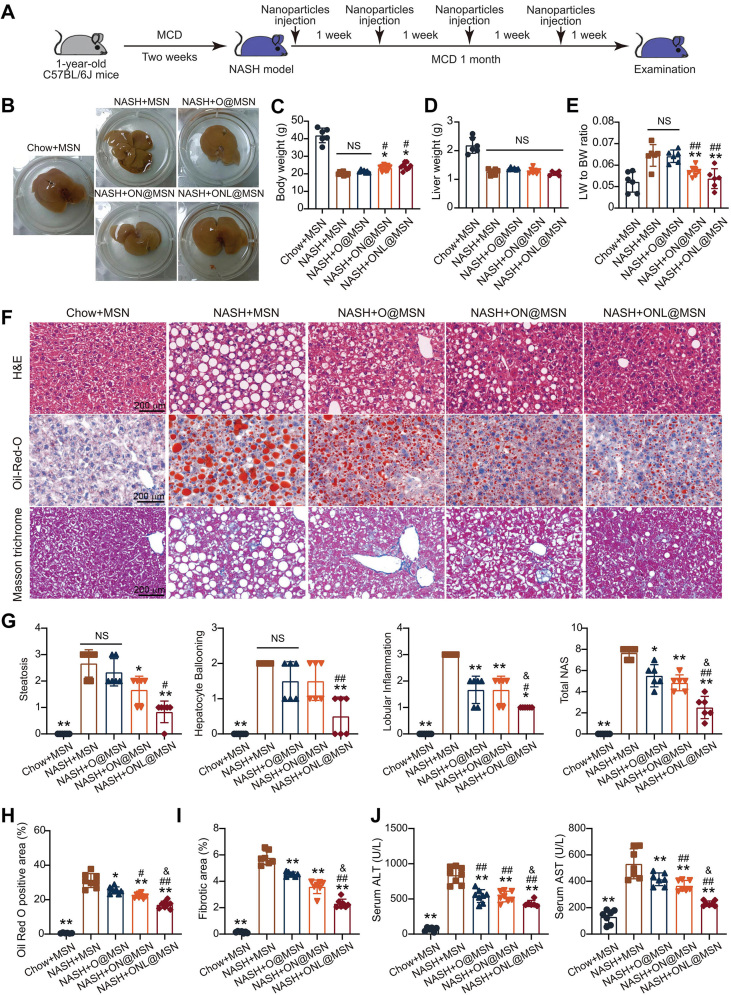
Figure 4.
ONL@MSN alleviates inflammation, steatosis, and fibrosis in a NASH mouse model. (A) Experimental design for the administration of nanoparticles in a mouse NASH model. (B) Representative images of liver tissue from different groups, including the mice treated with Chow + MSN (chow-fed mice receiving MSN injection), NASH + MSN group (NASH mice receiving MSN injection), NASH + O@MSN group (NASH mice receiving O@MSN injection), NASH + ON@MSN group (NASH mice receiving ON@MSN injection), and NASH + ONL@MSN group (NASH mice receiving ONL@MSN injection). (C–E) The effect of drug-loaded nanoparticle treatment on body weight (C), liver weight (D), and the liver weight-to-body weight ratio (E). (F) Representative images of liver sections stained with H&E, Oil Red O, and Masson's trichrome. Scale bar = 200 μm. (G) Statistical indexes of steatosis, hepatocyte ballooning, and lobular inflammation, and the calculated NAFLD activity score (NAS). (H) Quantification of lipid accumulation in the liver by Oil Red O staining. (I) Quantification of liver fibrosis by Masson's staining. (J) Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels in mice. Data are presented as mean ± SEM and analyzed using one-way ANOVA followed by Tukey's analysis. ∗P < 0.05, ∗∗P < 0.01 vs. NASH + MSN. #P < 0.05, ##P < 0.01 vs. NASH + O@MSN. &P < 0.05 vs. NASH + ON@MSN. NS, no significance. n = 6 per group.