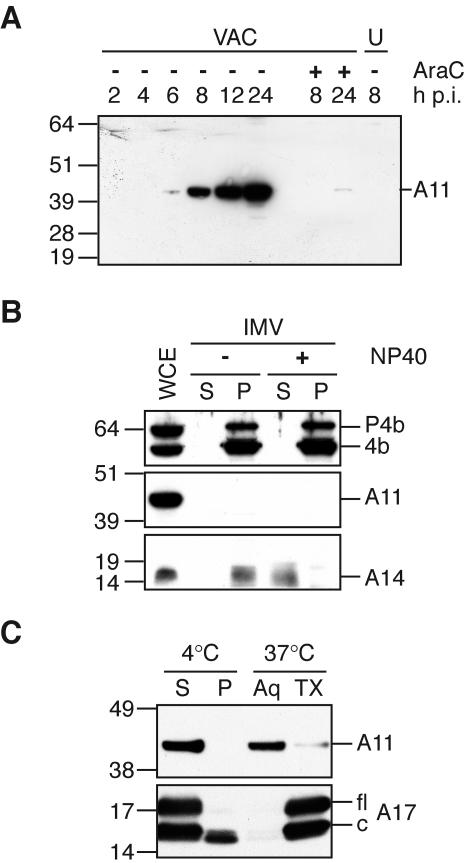
FIG. 2.
Synthesis of A11 and analysis of IMV. (A) A11 expression kinetics. BS-C-1 cells were infected with 10 PFU per cell of VAC and whole-cell extracts were prepared at the indicated times. Extracts from uninfected cells (U) and cells infected in the presence of cytosine arabinoside (AraC) were also prepared, and all extracts were analyzed by SDS-PAGE and Western blotting with a anti-A11 antiserum. The position and mass in kilodaltons of marker proteins are indicated on the left. Note that the apparent molecular mass of A11 is slightly larger than predicted. (B) Absence of A11 in purified IMV. Purified IMV was extracted with NP-40 (+) or mock treated (−) and separated into soluble (S) and pellet (P) fractions. Proteins in both fractions were separated by SDS-PAGE, followed by Western blotting with anti-P4b, anti-A11, or anti-A14 antisera. Whole-cell extract (WCE) containing similar amounts of P4b/4b and A14 was included in the analysis. The position and mass in kilodaltons of marker proteins are indicated on the left. (C) Phase separation of A11 in Triton X-114. BS-C-1 cells were infected with 3 PFU per cell of VAC. After 20 h, cells were harvested in cold lysis buffer containing 1% Triton X-114. The lysate was separated into soluble (S) and pellet (P) fractions, and the soluble fraction was separated into aqueous (Aq) and detergent (TX) phases. All samples were adjusted to equal volumes and subjected to SDS-PAGE, followed by Western blotting with anti-A11 or anti-A17 antisera. The position and mass in kilodaltons of marker proteins are indicated on the left. Lines on the right point to the full-length (fl) and cleaved (c) forms of the A17 protein.