Abstract
Murine gammaherpesvirus 68 (MHV-68) is a naturally occurring rodent pathogen with significant homology to human pathogens Epstein-Barr virus and Kaposi's sarcoma-associated herpesvirus. T cells are essential for primary clearance of MHV-68 and survival of mice following intranasal infection. Previous reports have suggested that protein kinase C θ (PKCθ) is essential for T-cell activation and cytokine production in vitro. To determine the role of this molecule in vivo during the immune response to a viral infection, PKCθ−/− mice were infected with MHV-68. Despite the essential role of T cells in viral clearance, PKCθ−/− mice survived infection, cleared lytic virus, and maintained effective long-term control of latency. CD8 T-cell expansion, trafficking to the lung, and cytotoxic activity were similar in PKCθ+/+ and PKCθ−/− mice, whereas antiviral antibody and T-helper cell cytokine production were significantly lower in PKCθ−/− mice than in PKCθ+/+ mice. These studies demonstrate a differential requirement for PKCθ in the immune response to MHV-68 and show that PKCθ is not essential for the T-cell activation events leading to viral clearance.
Protein kinase C θ (PKCθ) is an isoenzyme of the PKC family that is selectively expressed in T lymphocytes (2, 19, 20). In mature T cells, stimulation with antigen and CD28 induces PKCθ translocation from the cytosol into plasma membrane lipid rafts, where it colocalizes with T-cell receptor at the central core of the immune synapse (4, 21). PKCθ subsequently mediates activation of several transcription factors, including NF-κB, NFAT, and AP-1, resulting in T-cell activation and increased interleukin 2 (IL-2) gene expression (reviewed in references 1 and 11). Studies of cell lines have led to the conclusion that PKCθ is essential for T-cell activation and that this isoenzyme of PKC appears to function by integrating signals from CD28 and the T-cell receptor (reviewed in references 1 and 11). Studies in mice deficient in PKCθ have tended to support this view and have shown that PKCθ is critical for NF-κB activation in mature T lymphocytes (29), NFAT activation (22), pulmonary allergic hypersensitivity responses (17), TH2 responses to Nippostrongylus brasiliensis (17), and the induction of T-cell activation versus tolerance in vivo (3). However, recent studies have demonstrated T-cell responses to lymphocytic choriomeningitis virus (3) and Leishmania major (17) in PKCθ−/− mice, suggesting that some infectious agents may be able to overcome the requirement for PKCθ in T-cell activation.
To determine the role of PKCθ in T-cell activation during the immune response to MHV-68, we infected wild-type or PKCθ−/− mice with the virus, a rodent pathogen (5), which is closely related to Epstein-Barr virus and Kaposi's sarcoma-associated herpesvirus (8, 36). Intranasal administration of MHV-68 results in acute productive infection of lung alveolar epithelial cells and a latent infection in various cell types, including B lymphocytes (9, 10, 28, 30, 35, 37). The virus induces an inflammatory infiltrate in the lungs, splenomegaly, and an increase in the number of activated CD8 T cells in the blood (30, 33). Virus-specific CD8 T cells traffic to the lungs and clear infectious virus by a cytolytic mechanism 10 to 15 days after infection (30, 31), while both B and T cells appear to function in the long-term control of latent virus (12, 28).
Our previous studies (14) showed that CD28 is not essential for cytotoxic T-lymphocyte (CTL) responses to MHV-68 or for primary viral clearance. However, lack of CD28 resulted in a significant reduction in antiviral antibody titers. Furthermore, Kim et al. (12) demonstrated that the compromised antiviral antibody response in CD28−/− mice was ineffective in the long-term control of latent MHV-68, whereas T-cell responses remained effective. To further delineate signaling pathways in T-cell activation during MHV-68 infection, in the current study we utilized PKCθ−/− mice to determine whether T-cell activation and viral clearance were mediated via PKCθ in a CD28-independent pathway or whether an alternative PKCθ-independent pathway was utilized.
MATERIALS AND METHODS
Mice.
129/B6 mice that were heterozygous (PKCθ+/−) for the disruption of the PKCθ gene (29) were kindly provided by Amnon Altman, La Jolla Institute for Allergy and Immunology, San Diego, Calif., with the prior permission of Dan Littman, Skirball Institute, New York. Mice were bred and housed under specific-pathogen-free conditions in the vivarium at the La Jolla Institute for Allergy and Immunology or Torrey Pines Institute for Molecular Studies. PKCθ−/− homozygous knockout mice and PKCθ+/+ littermates were obtained from pairings of heterozygous mice. The genotypes of the progeny were determined by PCR on tail snips. Age-matched 6- to 15-week-old female PKCθ+/+ and PKCθ−/− mice were used in all experiments.
Viral infection and sampling.
MHV-68 (clone G2.4) (5, 30) was propagated in BHK-21 cells (ATCC CCL-10). Mice were anesthetized with Avertin (2,2,2-tribromoethanol) and infected intranasally with 105 PFU of the virus in phosphate-buffered saline. At various intervals after infection, the mice were terminally anesthetized with Avertin. The lungs were removed and homogenized in medium on ice prior to virus titration. Single-cell suspensions were prepared from the bronchoalveolar lavage (BAL) or spleen, and titers of replicating virus were determined by plaque assay on NIH 3T3 cells (ATCC CRL1658) as described previously (7). The detection limit of this assay is 10 PFU/ml of a 10% tissue homogenate based on data for plaques recovered from homogenates of uninfected tissues spiked with known amounts of virus.
Cytotoxicity assays.
Cytotoxic T-cell activity was determined using a redirected chromium release assay. Suspensions of spleen or BAL cells from MHV-68-infected animals were incubated with 51Cr-labeled FcR+ P815 (ATCC TIB-64) target cells in the presence of 2 μg/ml 2C11 anti-CD3 antibody for 6 to 8 h at 37°C as previously described (7). The level of specific 51Cr release is a measure of total (virus-specific and nonspecific) cytotoxicity.
Measurement of cytokine responses.
The frequency of MHV-68-specific CD8 T cells in spleen or BAL was determined by intracellular staining for gamma interferon (IFN-γ). Restimulation of spleen or BAL cells with MHV-68 peptides p56 (AGPHNDMEI from ORF6) and p79 (TSINFVKI from ORF61) and dual staining for IFN-γ and CD8 were performed as described by Stevenson et al. (26). These peptides have been identified as major epitopes (16, 26). Cytokine levels in culture supernatants from cells that had been restimulated in vitro with irradiated virus-infected splenic antigen-presenting cells were assayed by sandwich enzyme-linked immunosorbent assay (ELISA) as described previously (24). All reagents were obtained from BD PharMingen (San Diego, CA).
Flow cytometric analysis.
Cells were stained with phycoerythrin- or fluorescein isothiocyanate-conjugated monoclonal antibodies, as described previously (14). All antibodies were purchased from BD PharMingen (San Diego, CA). Isotype controls were included in each assay.
ELISA for virus-specific antibody.
Serum antibody titers were determined by ELISA, as described previously (15), using peroxidase-labeled secondary reagents from Southern Biotechnology (Birmingham, Ala.). Sera from uninfected mice and positive-control sera were included in each assay.
RESULTS
PKCθ−/− mice can clear replicating MHV-68 from their lungs and maintain effective long-term control of the virus.
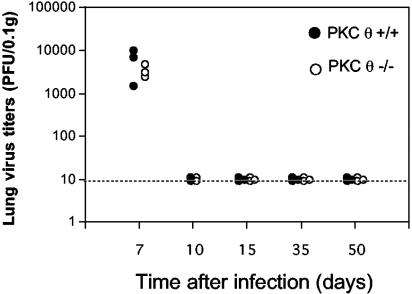
Despite the essential role of T cells in viral clearance, mice homozygous for a targeted disruption of the PKCθ gene were able to clear lytic MHV-68 with normal kinetics (Fig. 1). Both PKCθ−/− and PKCθ+/+ mice had cleared infectious virus from their lungs by day 10 after intranasal infection with 105 PFU MHV-68. Thus, in this in vivo model, PKCθ did not appear to be essential for the T-cell activation events leading to viral clearance. The lungs of both PKCθ−/− and PKCθ+/+ mice remained clear of replicating virus at days 35 and 50 after infection (Fig. 1), demonstrating effective long-term control of the virus.
FIG. 1.
Lung virus titers in PKCθ+/+ and PKCθ−/− mice. PKCθ−/− or PKCθ+/+ mice were infected intranasally with 105 PFU MHV-68. At various times after infection, lungs were harvested and virus titers determined in lung homogenates by plaque assay. Data are expressed as PFU/0.1 g lung tissue for individual mice.
PKCθ is not required for CD8 T-cell expansion or trafficking to the lungs.
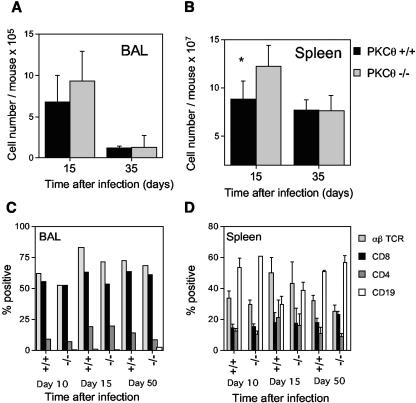
Intranasal infection with MHV-68 induces an inflammatory infiltrate in the lungs comprising mainly T cells and monocytes/macrophages. There was no significant difference in the total number of cells recovered in the BAL in PKCθ−/− and PKCθ+/+ mice (Fig. 2A) or in the percentages of CD4 and CD8 T cells in this population determined by flow cytometric analysis (Fig. 2C). In both PKCθ−/− and PKCθ+/+ mice, CD8 T cells greatly outnumbered the CD4 T cells. MHV-68 also induces splenomegaly by a mechanism that is dependent on both T and B cells (7, 9, 34, 35). Lack of PKCθ did not diminish MHV-68-induced splenomegaly (Fig. 2B). In contrast, splenic cellularity was significantly increased in PKCθ−/− mice on day 15 after infection. However, this appeared to be a temporary phenomenon, and the cell numbers were similar in PKCθ−/− and PKCθ+/+ mice by day 35 after infection (Fig. 2B). The percentages of CD4 and CD8 T cells and B cells were similar in the spleens of PKCθ−/− and PKCθ+/+ mice (Fig. 2D). The cell numbers and phenotypes were similar in the spleens of uninfected PKCθ−/− and PKCθ+/+ mice (data not shown).
FIG. 2.
Cell numbers and lymphocyte subsets in the BAL or spleens of PKCθ+/+ and PKCθ−/− mice. The numbers of cells in the BAL (A) or spleen (B) were determined at intervals after intranasal infection of PKCθ+/+ and PKCθ−/− mice with MHV-68. Data are means ± standard deviations (error bars) of cell counts for two separate experiments on cells 15 days after infection and for one experiment on cells 35 days after infection. Cell counts from three or four individual mice were performed at each time point. BAL (C) or spleen cells (D) were stained with phycoerythrin- or fluorescein isothiocyanate-conjugated monoclonal antibodies, and the resulting populations were analyzed by flow cytometry. The means ± standard deviations (error bars) of data from two separate experiments on cells 15 days after infection and one experiment each on cells 10 and 35 days postinfection are shown. Groups of two to four mice were used at each time point. BAL cells were pooled in each experiment, whereas individual spleen cell suspensions were analyzed. The asterisk denotes that there was a statistically significant difference in spleen cell numbers at day 15 after infection in PKCθ−/− and PKCθ+/+ mice (P < 0.05 by Student's t test).
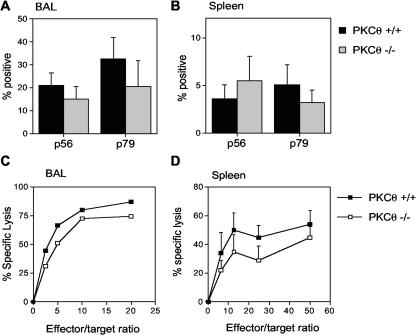
The proportion of virus-specific CD8 T cells in the BAL and spleen was also determined by evaluating the number of CD8 cells producing IFN-γ in response to stimulation with two different viral peptide epitopes by intracellular staining. There was no significant difference in the percentages of CD8 T cells responding to the p56 and p79 viral epitopes in the BAL (Fig. 3A) or in the spleens of infected animals (Fig. 3B). Taken together, these data indicate that CD8 T cells from PKCθ−/− mice are capable of expansion and trafficking to an inflammatory site in response to MHV-68 infection.
FIG. 3.
CD8 T-cell responses in PKCθ−/− mice. Frequency of virus-specific CD8 T cells in BAL (A) and spleen (B). BAL or spleen cells were harvested 15 days after infection and stimulated for 6 h with either p56 or p79 MHV-68 peptide in the presence of monensin prior to staining for CD8 and IFN-γ. Results are means ± standard deviations (error bars) for two separate experiments. Cell suspensions from three or four mice were analyzed individually in each experiment. CTL responses in BAL (C) and spleen (D). Single-cell suspensions were prepared from BAL or spleens at day 10 after intranasal infection with MHV-68. CTL activity was determined in a 6-h 51Cr release assay. Mean percentages of specific lysis ± standard deviations (error bars) are shown for two separate experiments. Groups of two or three mice were used in each experiment. BAL cells were pooled in each experiment, whereas spleen cell suspensions were analyzed individually.
PKCθ is not required for the development of CTL responses during MHV-68 infection.
MHV-68 is cleared from the lungs by cytotoxic T cells 10 to 13 days after infection (31). The normal kinetics of viral clearance in PKCθ−/− mice suggested an intact CTL response. To verify this, CTL assays were performed on BAL and spleen cell populations from infected animals. Previous studies in this model have shown that the majority of the CTL activity is mediated by CD8 T cells (7, 31). There was no significant difference in CTL activity in either the spleen or BAL from PKCθ−/− and PKCθ+/+ mice (Fig. 3C and D). Therefore, PKCθ is not essential for the CTL response to MHV-68 infection.
Reduced T-helper cell cytokine responses in PKCθ−/− mice.
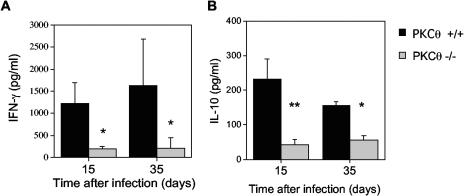
To determine the effect of absence of PKCθ on cytokine production during the immune response to viral infection, splenocytes from virus-infected mice were restimulated in vitro with virus-infected splenic antigen-presenting cells as described previously (24). Recall cytokine production was measured in the supernatant by ELISA. IFN-γ production was dramatically reduced in splenocytes from PKCθ−/− mice (Fig. 4A). Subset depletion experiments have shown that CD4 T cells are the predominant cell type producing IFN-γ in these cultures (14). There was also a significant reduction in IL-10 production (Fig. 4B) while no IL-2, IL-4, or IL-5 was detected in the culture supernatants of cells from either PKCθ+/+ or PKCθ−/− mice (data not shown).
FIG. 4.
Cytokine responses to MHV-68 in PKCθ−/− mice. Spleens were harvested 15 or 35 days after infection with MHV-68. Splenocytes were restimulated with MHV-68-infected antigen-presenting cells, and IFN-γ (A) or IL-10 (B) concentrations were determined in culture supernatants by ELISA. Data are expressed as mean cytokine concentrations (pg/ml) ± standard deviations (error bars) for two separate experiments of cells, one 15 days after infection and one 35 days after infection. Splenocyte cultures from two to four individual PKCθ−/− or PKCθ+/+ mice were tested in each experiment. Asterisks denote that there was a statistically significant difference in cytokine responses in PKCθ−/− and PKCθ+/+ mice (P < 0.05 [*] and P < 0.01 [**] by the Mann-Whitney rank sum test).
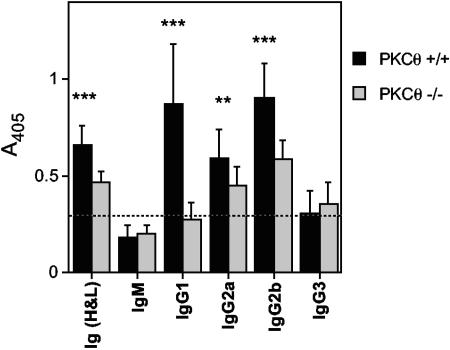
Reduced serum antibody responses to MHV-68 in PKCθ−/− mice.
Substantial changes in the antibody response to MHV-68 were also noted in PKCθ−/− mice. Virus-specific serum antibody titers were determined by ELISA 50 days after infection with MHV-68. There was a significant reduction in the level of virus-specific antibody in PKCθ−/− mice compared to that in wild-type mice (Fig. 5). There was a more dramatic reduction in immunoglobulin G1 (IgG1) than in IgG2a or IgG2b. Virus-specific IgM and IgG3 were detected at low levels in both PKCθ+/+ and PKCθ−/− mice. Similar observations were made at days 15 and 35 after infection (data not shown). Thus, like CD28−/− mice, PKCθ−/− mice can mount humoral responses to MHV-68, and class switching still occurs. However, the response is significantly reduced compared to that in wild-type mice.
FIG. 5.
MHV-68-specific antibody responses in PKCθ−/− mice. Serum samples were collected from PKCθ−/− mice 50 days after infection with MHV-68. Virus-specific antibody responses were determined by ELISA. Data are expressed as mean absorbance values ± standard deviations (error bars) for two separate experiments. Sera from four or five individual mice were tested in each experiment. Asterisks denote that there was a statistically significant difference in antibody titers in PKCθ−/− and PKCθ+/+ mice (P < 0.01 [**] and P < 0.001 [***] by Student's t test). Ig (H&L), heavy and light chains of Ig.
DISCUSSION
Data from previous in vitro studies (reviewed in references 1 and 11) have led to the conclusion that PKCθ is essential for T-cell activation, and it is widely accepted that PKCθ functions as a pivotal convergence point for pathways of T-cell activation. As T-cell activation is a prerequisite for the clearance of MHV-68, in the current study we examined the role of PKCθ in the immune response to this virus. In contrast with the previous in vitro studies, we showed that PKCθ is not essential for the T-cell activation events required for clearance of MHV-68. Our data show that both PKCθ-dependent and -independent pathways of T-cell activation operate during the immune response to a viral infection. Thus, in our in vivo model, normal expansion of CD8 T cells occurred in response to MHV-68 infection, and similar frequencies of CD8 T cells responding to major viral epitopes were detected. In addition, virus-specific CD8 T cells were able to migrate successfully to the lungs in PKCθ−/− mice, indicating that upregulation of the required chemokine receptors and adhesion molecules was not dependent on PKCθ. Clearance of lytic MHV-68 from the lungs of wild-type mice is mediated primarily by CD8 T cells via a cytolytic mechanism (7, 31). In accordance with the ability of PKCθ−/− mice to clear infectious MHV-68 from the lungs with normal kinetics, CTL activity was not significantly affected in these mice. In this respect, PKCθ−/− mice resemble CD28−/− mice, which are able to clear lytic MHV-68 from the lungs (12, 14). Also, like CD28−/− mice, PKCθ−/− mice are able to maintain effective long-term control of latent MHV-68, whereas mice lacking CD4 T cells, CD40, or CD40L show viral reactivation in the lungs (6, 7, 14, 25). In the case of CD28−/− mice, it has been shown that T cells are the effector cells maintaining long-term control of latent MHV-68 (12), whereas both antibody and T-cell responses are compromised in mice lacking CD4 or CD40 costimulation (6, 7, 25).
Our preliminary data (M. D. Wareing and S. R. Sarawar, unpublished) suggest that the nonessential role of PKCθ in viral clearance is not limited to MHV-68, as PKCθ−/− mice also survive infection with influenza virus, whereas T-cell-deficient mice rapidly succumb to both viruses. Like MHV-68, influenza virus is cleared from the lungs by cytotoxic T cells (32). Furthermore, in a recent study, Berg-Brown et al. (3) were also able to demonstrate a CTL response to lymphocytic choriomeningitis virus in virally infected PKCθ−/− mice. Our studies are in agreement with this finding and, in addition, show that the CTL response to MHV-68 is effective in mediating viral clearance. Thus, collectively, these studies demonstrate that viral infections can circumvent the requirement for PKCθ in CTL activation.
However, not all aspects of T-cell function in response to MHV-68 infection were independent of PKCθ. We showed that T-helper cell cytokine production following MHV-68 infection was significantly reduced in PKCθ−/− mice compared to that in wild-type mice. Thus, we observed a dramatic reduction in IFN-γ production, which was sustained in PKCθ−/− mice, whereas our previous studies (14) showed only a temporary reduction in IFN-γ production in CD28−/− mice. This suggests a role for PKCθ beyond CD28 signaling in cytokine production. We did not observe a switch to a TH2 profile in PKCθ−/− mice, and no IL-4 or IL-5 was detected in cultures from either wild-type or PKCθ−/− mice. However, the effect was not limited to TH1 cytokines, as IL-10 production was also reduced. Recently, Marsland et al. (17) reported a decrease in TH2 responses to ovalbumin and Leishmania in PKCθ−/− mice. However, IFN-γ responses were intact in these models, in contrast to our current findings, suggesting that the nature of the antigenic stimulus may dictate the dependence of TH1 responses on PKCθ.
The present study also showed that there was a significant reduction in antiviral serum antibody titers in PKCθ−/− mice. Since PKCθ is expressed in both CD4 and CD8 T cells, whereas little or none is expressed in B cells (2, 19, 20), this likely reflects a defect in CD4 T-cell help. CD28−/− mice also show a significant reduction in anti-MHV-68 serum titers (12, 14). Interestingly, Kim et al. (12) showed that the compromised antibody response in CD28−/− mice was ineffective in preventing reactivation of latent MHV-68 in the lungs, whereas the antibody response in wild-type mice controlled the virus effectively. They showed that it was the T-cell response that maintained effective control of latency in CD28−/− mice (12). It is likely that this is also the case in PKCθ−/− mice, although it is possible that the reduced antibody response is still capable of exerting some control of latency. Our data showed that lack of PKCθ had a more dramatic effect on IgG1 than IgG2b or IgG2a. A similar differential effect on antiviral IgG1 versus IgG2 was noted following MHV-68 infection of CD28−/− mice in our earlier study (14) and by McAdam et al. (18) in vesicular stomatitis virus (VSV)-infected CD28−/− mice. However, Berg-Brown et al. (3) reported that PKCθ was not required for an in vitro neutralizing antibody response to VSV, although they did not examine the subclass distribution of the antiviral antibody response. The reason for this difference in the PKCθ dependence of the antibody response during MHV-68 and VSV infection is not readily apparent. However, the antibody responses are somewhat different in the two viral infections. The helper-dependent IgG response develops much more rapidly following infection with VSV (3) than with MHV-68 (23, 27). It is possible that VSV is able to trigger costimulatory molecules that utilize PKCθ-independent signaling pathways for B-cell help, while MHV-68 may be unable to trigger such signals.
The reason that PKCθ is dispensable for certain T-cell functions during a viral infection in vivo but is required for T-cell activation in vitro is currently unclear. However, similar disparities between the in vitro and in vivo requirements for T-cell activation have been observed in other systems. For example, studies in IL-2−/− mice showed that, although IL-2 is essential for T-cell proliferation in vitro, in vivo it does not appear to be required for T-cell expansion and CTL activity in response to viral infection (13). This likely reflects substitution of IL-2 function in vivo by other cytokines that also signal through the common gamma chain and by the ability of viruses to trigger costimulatory events. Viral infection activates the innate immune system, leading to the production of proinflammatory cytokines and other mediators, while viral components can act as ligands for cell surface receptors. These events can induce potent NF-κB activation, and it is possible that PKCθ-independent NF-κB activation, triggered by innate immune responses, can partially compensate for the lack of PKCθ in adaptive immunity to viruses.
In summary, our data provide novel insights into the role of PKCθ in T-cell activation during the immune response to a viral infection. No previous studies have examined T-cell trafficking, cytokine production, or antiviral IgG subclass distribution during a viral infection in CD28−/− mice. Furthermore, no previous studies have examined viral clearance or the long-term control of persistent virus in CD28−/− mice. Our data show that PKCθ is not universally required for T-cell activation in vivo—rather it is differentially required for different arms of the immune response. The idea that distinct signaling pathways, not all of which converge at the level of PKCθ, regulate T-cell activity, suggests that it may be possible to selectively block certain T-cell functions while preserving others. In this respect, it of interest that Marsland et al. (17) have recently shown that PKCθ is required for pulmonary allergic inflammation. Taken together with our findings, these data suggest further evaluation of PKCθ as a possible target for novel immunosuppressive drugs that could block T-cell functions leading to lung damage, without compromising viral clearance.
Acknowledgments
This work was supported in part by a grant from the Infectious Disease Science Center.
We thank Amnon Altman for helpful advice and discussion and Svetlana Lebedeva and Erin Campbell for assistance with the maintenance and genotyping of the PKCθ mice.
REFERENCES
- 1.Arendt, C. W., B. Albrecht, T. J. Soos, and D. R. Littman. 2002. Protein kinase C-theta: signaling from the center of the T-cell synapse. Curr. Opin. Immunol. 14:323-330. [DOI] [PubMed] [Google Scholar]
- 2.Baier, G., D. Telford, L. Giampa, K. M. Coggeshall, G. Baier-Bitterlich, N. Isakov, and A. Altman. 1993. Molecular cloning and characterization of PKC theta, a novel member of the protein kinase C (PKC) gene family expressed predominantly in hematopoietic cells. J. Biol. Chem. 268:4997-5004. [PubMed] [Google Scholar]
- 3.Berg-Brown, N. N., M. A. Gronski, R. G. Jones, A. R. Elford, E. K. Deenick, B. Odermatt, D. R. Littman, and P. S. Ohashi. 2004. PKCθ signals activation versus tolerance in vivo. J. Exp. Med. 199:743-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bi, K., Y. Tanaka, N. Coudronniere, K. Sugie, S. Hong, M. J. van Stipdonk, and A. Altman. 2001. Antigen-induced translocation of PKC-theta to membrane rafts is required for T cell activation. Nat. Immunol. 2:556-563. [DOI] [PubMed] [Google Scholar]
- 5.Blaskovic, D., M. Stancekova, J. Svobodova, and J. Mistrikova. 1980. Isolation of five strains of herpesviruses from two species of free living small rodents. Acta Virol. 24:468. [PubMed] [Google Scholar]
- 6.Brooks, J. W., A. M. Hamilton-Easton, J. P. Christensen, R. D. Cardin, C. L. Hardy, and P. C. Doherty. 1999. Requirement for CD40 ligand, CD4+ T cells, and B cells in an infectious mononucleosis-like syndrome. J. Virol. 73:9650-9654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardin, R. D., J. W. Brooks, S. R. Sarawar, and P. C. Doherty. 1996. Progressive loss of CD8+ T cell-mediated control of a gammaherpesvirus in the absence of CD4+ T cells. J. Exp. Med. 184:863-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Efstathiou, S., Y. M. Ho, S. Hall, C. J. Styles, S. D. Scott, and U. A. Gompels. 1990. Murine herpesvirus 68 is genetically related to the gammaherpesviruses Epstein-Barr virus and herpesvirus saimiri. J. Gen. Virol. 71:1365-1372. [DOI] [PubMed] [Google Scholar]
- 9.Ehtisham, S., N. P. Sunil-Chandra, and A. A. Nash. 1993. Pathogenesis of murine gammaherpesvirus infection in mice deficient in CD4 and CD8 T cells. J. Virol. 67:5247-5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flano, E., S. M. Husain, J. T. Sample, D. L. Woodland, and M. A. Blackman. 2000. Latent murine gamma-herpesvirus infection is established in activated B cells, dendritic cells, and macrophages. J. Immunol. 165:1074-1081. [DOI] [PubMed] [Google Scholar]
- 11.Isakov, N., and A. Altman. 2002. Protein kinase Cθ in T cell activation. Annu. Rev. Immunol. 20:761-794. [DOI] [PubMed] [Google Scholar]
- 12.Kim, I. J., E. Flano, D. L. Woodland, and M. A. Blackman. 2002. Antibody-mediated control of persistent gamma-herpesvirus infection. J. Immunol. 168:3958-3964. [DOI] [PubMed] [Google Scholar]
- 13.Kundig, T. M., H. Schorle, M. F. Bachmann, H. Hengartner, R. M. Zinkernagel, and I. Horak. 1993. Immune responses in interleukin-2-deficient mice. Science 262:1059-1061. [DOI] [PubMed] [Google Scholar]
- 14.Lee, B. J., S. K. Reiter, M. Anderson, and S. R. Sarawar. 2002. CD28−/− mice show defects in cellular and humoral immunity but are able to control infection with murine gammaherpesvirus 68. J. Virol. 76:3049-3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee, B. J., S. Santee, S. Von Gesjen, C. F. Ware, and S. R. Sarawar. 2000. Lymphotoxin-alpha-deficient mice can clear a productive infection with murine gammaherpesvirus 68 but fail to develop splenomegaly or lymphocytosis. J. Virol. 74:2786-2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu, L., E. Flano, E. J. Usherwood, S. Surman, M. A. Blackman, and D. L. Woodland. 1999. Lytic cycle T cell epitopes are expressed in two distinct phases during MHV-68 infection. J. Immunol. 163:868-874. [PubMed] [Google Scholar]
- 17.Marsland, B. J., T. J. Soos, G. Spath, D. R. Littman, and M. Kopf. 2004. Protein kinase C theta is critical for the development of in vivo T helper (Th)2 cell but not Th1 cell responses. J. Exp. Med. 200:181-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McAdam, A. J., E. A. Farkash, B. E. Gewurz, and A. H. Sharpe. 2000. B7 costimulation is critical for antibody class switching and CD8+ cytotoxic T-lymphocyte generation in the host response to vesicular stomatitis virus. J. Virol. 74:203-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meller, N., A. Altman, and N. Isakov. 1998. New perspectives on PKCθ, a member of the novel subfamily of protein kinase C. Stem Cells 16:178-192. [DOI] [PubMed] [Google Scholar]
- 20.Mischak, H., J. Goodnight, D. W. Henderson, S. Osada, S. Ohno, and J. F. Mushinski. 1993. Unique expression pattern of protein kinase C-theta: high mRNA levels in normal mouse testes and in T-lymphocytic cells and neoplasms. FEBS Lett. 326:51-55. [DOI] [PubMed] [Google Scholar]
- 21.Monks, C. R., H. Kupfer, I. Tamir, A. Barlow, and A. Kupfer. 1997. Selective modulation of protein kinase C-theta during T-cell activation. Nature 385:83-86. [DOI] [PubMed] [Google Scholar]
- 22.Pfeifhofer, C., K. Kofler, T. Gruber, N. G. Tabrizi, C. Lutz, K. Maly, M. Leitges, and G. Baier. 2003. Protein kinase C theta affects Ca2+ mobilization and NFAT cell activation in primary mouse T cells. J. Exp. Med. 197:1525-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sangster, M. Y., D. J. Topham, S. D'Costa, R. D. Cardin, T. N. Marion, L. K. Myers, and P. C. Doherty. 2000. Analysis of the virus-specific and nonspecific B cell response to a persistent B-lymphotropic gammaherpesvirus. J. Immunol. 164:1820-1828. [DOI] [PubMed] [Google Scholar]
- 24.Sarawar, S. R., R. D. Cardin, J. W. Brooks, M. Mehrpooya, R. A. Tripp, and P. C. Doherty. 1996. Cytokine production in the immune response to murine gammaherpesvirus 68. J. Virol. 70:3264-3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarawar, S. R., B. J. Lee, S. K. Reiter, and S. P. Schoenberger. 2001. Stimulation via CD40 can substitute for CD4 T cell function in preventing reactivation of a latent herpesvirus. Proc. Natl. Acad. Sci. USA 98:6325-6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stevenson, P. G., G. T. Belz, J. D. Altman, and P. C. Doherty. 1999. Changing patterns of dominance in the CD8+ T cell response during acute and persistent murine gamma-herpesvirus infection. Eur. J. Immunol. 29:1059-1067. [DOI] [PubMed] [Google Scholar]
- 27.Stevenson, P. G., and P. C. Doherty. 1998. Kinetic analysis of the specific host response to a murine gammaherpesvirus. J. Virol. 72:943-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stewart, J. P., E. J. Usherwood, A. Ross, H. Dyson, and T. Nash. 1998. Lung epithelial cells are a major site of murine gammaherpesvirus persistence. J. Exp. Med. 187:1941-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun, Z., C. W. Arendt, W. Ellmeier, E. M. Schaeffer, M. J. Sunshine, L. Gandhi, J. Annes, D. Petrzilka, A. Kupfer, P. L. Schwartzberg, and D. R. Littman. 2000. PKC-theta is required for TCR-induced NF-κB activation in mature but not immature T lymphocytes. Nature 404:402-407. [DOI] [PubMed] [Google Scholar]
- 30.Sunil-Chandra, N. P., S. Efstathiou, J. Arno, and A. A. Nash. 1992. Virological and pathological features of mice infected with murine gamma-herpesvirus 68. J. Gen. Virol. 73:2347-2356. [DOI] [PubMed] [Google Scholar]
- 31.Topham, D. J., R. C. Cardin, J. P. Christensen, J. W. Brooks, G. T. Belz, and P. C. Doherty. 2001. Perforin and Fas in murine gammaherpesvirus-specific CD8+ T cell control and morbidity. J. Gen. Virol. 82:1971-1981. [DOI] [PubMed] [Google Scholar]
- 32.Topham, D. J., R. A. Tripp, and P. C. Doherty. 1997. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J. Immunol. 159:5197-5200. [PubMed] [Google Scholar]
- 33.Tripp, R. A., A. M. Hamilton-Easton, R. D. Cardin, P. Nguyen, F. G. Behm, D. L. Woodland, P. C. Doherty, and M. A. Blackman. 1997. Pathogenesis of an infectious mononucleosis-like disease induced by a murine gamma-herpesvirus: role for a viral superantigen? J. Exp. Med. 185:1641-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Usherwood, E. J., A. J. Ross, D. J. Allen, and A. A. Nash. 1996. Murine gammaherpesvirus-induced splenomegaly: a critical role for CD4 T cells. J. Gen. Virol. 77:627-630. [DOI] [PubMed] [Google Scholar]
- 35.Usherwood, E. J., J. P. Stewart, K. Robertson, D. J. Allen, and A. A. Nash. 1996. Absence of splenic latency in murine gammaherpesvirus 68-infected B cell-deficient mice. J. Gen. Virol. 77:2819-2825. [DOI] [PubMed] [Google Scholar]
- 36.Virgin, H. W., IV, P. Latreille, P. Wamsley, K. Hallsworth, K. E. Weck, A. J. Dal Canto, and S. H. Speck. 1997. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J. Virol. 71:5894-5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weck, K. E., S. S. Kim, H. I. Virgin, and S. H. Speck. 1999. Macrophages are the major reservoir of latent murine gammaherpesvirus 68 in peritoneal cells. J. Virol. 73:3273-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]