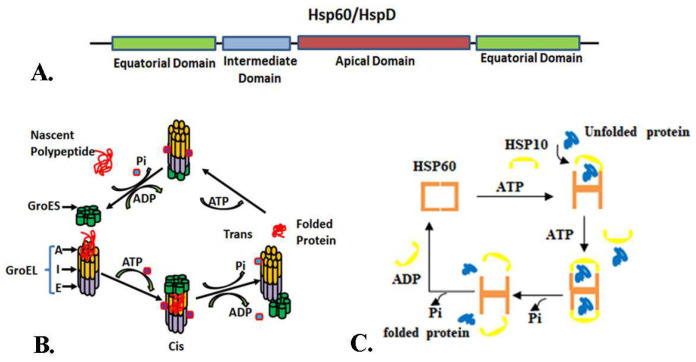
Figure 1.
The diagram illustrates the domain arrangement of the HSP60 protein, showcasing its bacterial form, GroEL, and its eukaryotic counterpart, HSP60. (A) The HSP60/GroEL structure features three principal domains: equatorial, intermediate, and apical. This protein folding process involves ATP-dependent folding of newly synthesized or misfolded proteins, facilitated by GroEL/GroES in bacteria (B) and HSP60/HSP10 in humans (C). ATP activation plays a crucial role in transitioning proteins between cis and trans conformations. Upon ATP hydrolysis, GroES/HSP10 dissociates from GroEL/HSP60, allowing the release of properly folded proteins into the cytoplasm.