Abstract
Most Pacific salmon species grow in the ocean, return to their native rivers to reproduce, and then die (semelparous type). However, rainbow trout survive after spawning and reproduce repeatedly until the end of their lives (iteroparous type). Little is known about how germline stem cells behave during gametogenesis in the two types of Pacific salmon. In this study, we show that all germline stem cells disappear after the first gametogenesis in Chinook and Kokanee salmon, whereas germline stem cells are maintained in rainbow trout. However, the germline stem cells of Chinook and Kokanee salmon transplanted into rainbow trout survive even after their spawning seasons and supply salmon gametes for multiple years. These results indicate that the behavior of the germline stem cells is mainly regulated by the somatic environment.
Germline stem cells disappear after the 1st spawning in salmon but supply gametes for multiple years when transplanted into trout.
INTRODUCTION
Most Pacific salmon, belonging to the genus Oncorhynchus, are known to spawn and spend their early life in fresh water, migrate to the sea, grow for several years in the ocean, return to their native river at age 3 to 7 years upon maturation, and die soon after spawning there (1). Unlike many other groups of fish, these species generally do not sexually mature and ovulate multiple times during the spawning season, and the oocytes in the ovaries develop in complete synchrony and eventually ovulate simultaneously (2). For males, spermatogenesis and spermiogenesis are known to occur almost synchronously, although the sperm that are released in the ductus deferens are used for several matings (3, 4). On the other hand, rainbow trout Oncorhynchus mykiss and cutthroat Oncorhynchus clarki, which are considered primitive species belonging to the same genus Oncorhynchus, return to their native river when they sexually mature and continue to live after spawning there, repeatedly producing gametes every year when the spawning season comes until the end of their lives (5, 6). Furthermore, rainbow trout and cutthroats have many land-locked populations, and, in these cases, egg formation and spermatogenesis are also repeated annually, as described above, and they do not die after the spawning seasons. Sockeye salmon Oncorhynchus nerka, a typical single spawning species, is also known to have many land-locked populations and is referred to as Kokanee salmon. These land-locked populations are also completely single-spawning, and both sexes are known to die after the first spawning season (7).
As described above, the single-spawning mode of reproduction seen in many Pacific salmon is called semelparity, while the multiple-spawning mode of reproduction seen in rainbow trout and cutthroat is called iteroparity. However, little is known about how these dimorphisms occur among closely related species within the genus Oncorhynchus. Although there have been several histological analyses of the gonads of these fish species (3, 8–11), the main concern in previous studies has been the cellular behavior of germ cells at their most differentiated and advanced stages in terms of egg and sperm production used for in vitro fertilization, and little has been described regarding the behavior of the most immature germ cells, so-called germline stem cells. Germline stem cells are expected to be important for iteroparity because they continuously provide a large number of gametes during the second and subsequent spawning seasons.
In this study, Chinook salmon Oncorhynchus tshawytscha and Kokanee salmon were used as models of semelparous species, and rainbow trout were used as a model of iteroparous species. First, by clarifying the behavior of undifferentiated germ cells (a population expected to contain stem cells) in the gonads of these species reared under the same conditions, we determined whether germline stem cells remain in the gonads of semelparous species immediately after the species spawn, i.e., whether the germline stem cells remain in the gonads to supply gametes during the following spawning seasons. This study revealed that germline stem cell behavior differed significantly between iteroparous and semelparous species. This difference in germline stem cell behavior raises the question of how germline stem cell behavior is regulated. Therefore, to elucidate whether the behavior of germline stem cells in these Pacific salmon is under cell-autonomous control or governed by the cellular environment, we tested whether (when germline stem cells of semelparous species were transplanted into rainbow trout, an iteroparous species) the recipient rainbow trout could continue producing gametes derived from the semelparous species for multiple years.
RESULTS
Behavior of undifferentiated germ cells
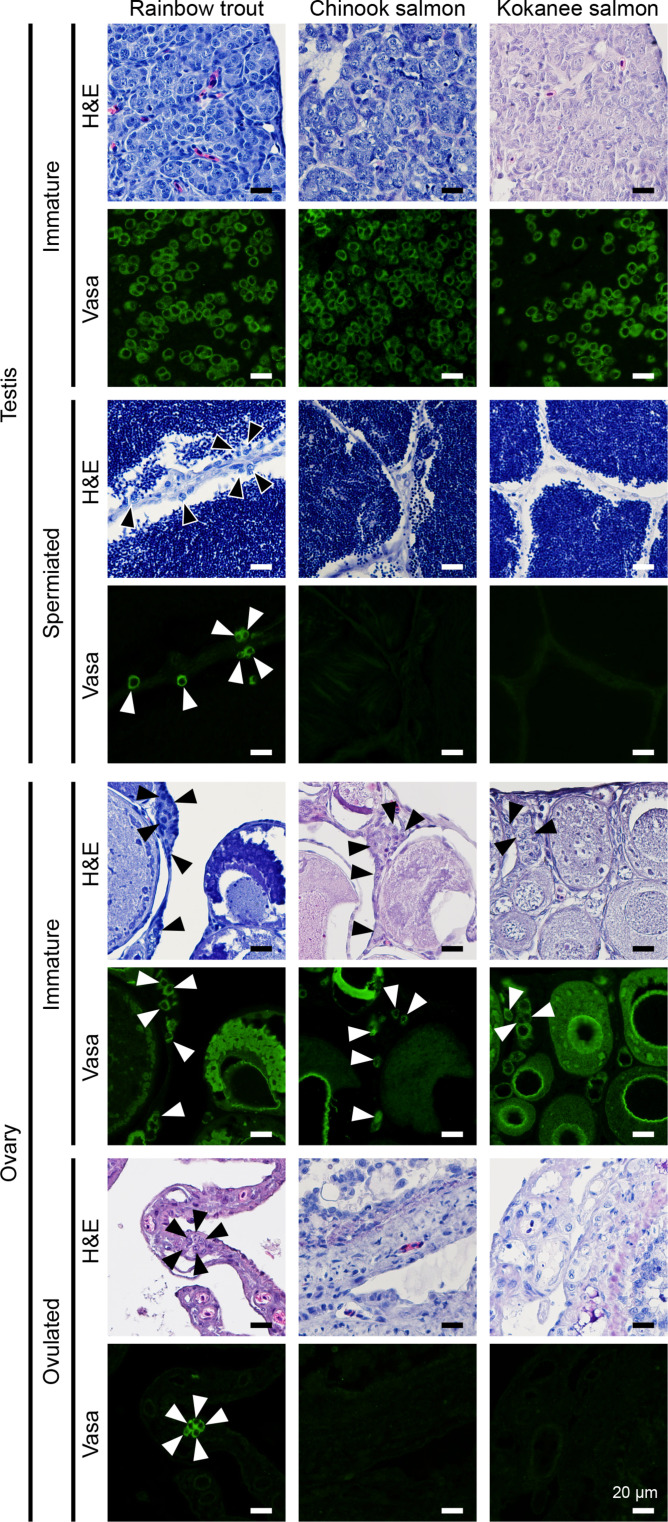
Because a reliable molecular marker for germline stem cells in salmonids has not yet been identified, we identified an undifferentiated germ cell population, including germline stem cells, by immunostaining with an antibody against Vasa, which is known to be strongly expressed in type A spermatogonia (ASGs) (12), oogonia, and relatively small oocytes (13). ASGs are expected to be undifferentiated and known to be singly located and surrounded by Sertoli cells. As a result, only Vasa-positive ASGs were observed in the prepubertal testes of the three salmonid species (Fig. 1, top). Similarly, in prepubertal ovaries, Vasa-positive oogonia were found in the ovarian epithelium located on the surface of the ovarian lamella in all examined fish species (Fig. 1, bottom). However, in the mature and spermiated testes, Vasa-positive ASGs were present within the wall of the testicular lobule only in rainbow trout, which is iteroparous, while no Vasa-positive cells could be detected in the two semelparous species (Fig. 1, top). Similarly, Vasa-positive oogonia could be detected in the postovulatory ovaries only in rainbow trout, but no Vasa-positive cells could be detected in the two semelparous salmon (Fig. 1, bottom). Thus, the two semelparous species have gonads that do not have the potencies to resume gametogenesis after their first reproductive cycle.
Fig. 1. Undifferentiated germ cells of rainbow trout, which are iteroparous, remain in the mature testes and ovulated ovaries, while undifferentiated germ cells of semelparous salmon (Chinook and Kokanee salmon) disappear with their maturation.
Left column, rainbow trout; middle column, Chinook salmon; right column, Kokanee salmon. Arrowheads indicate oogonia and spermatogonia in the ovary and the testis, respectively. The top and bottom pictures show hematoxylin and eosin (H&E)–stained and immunostained images using Vasa antibody, respectively. Scale bars, 20 μm.
Behavior of Chinook salmon germ cells in trout recipients
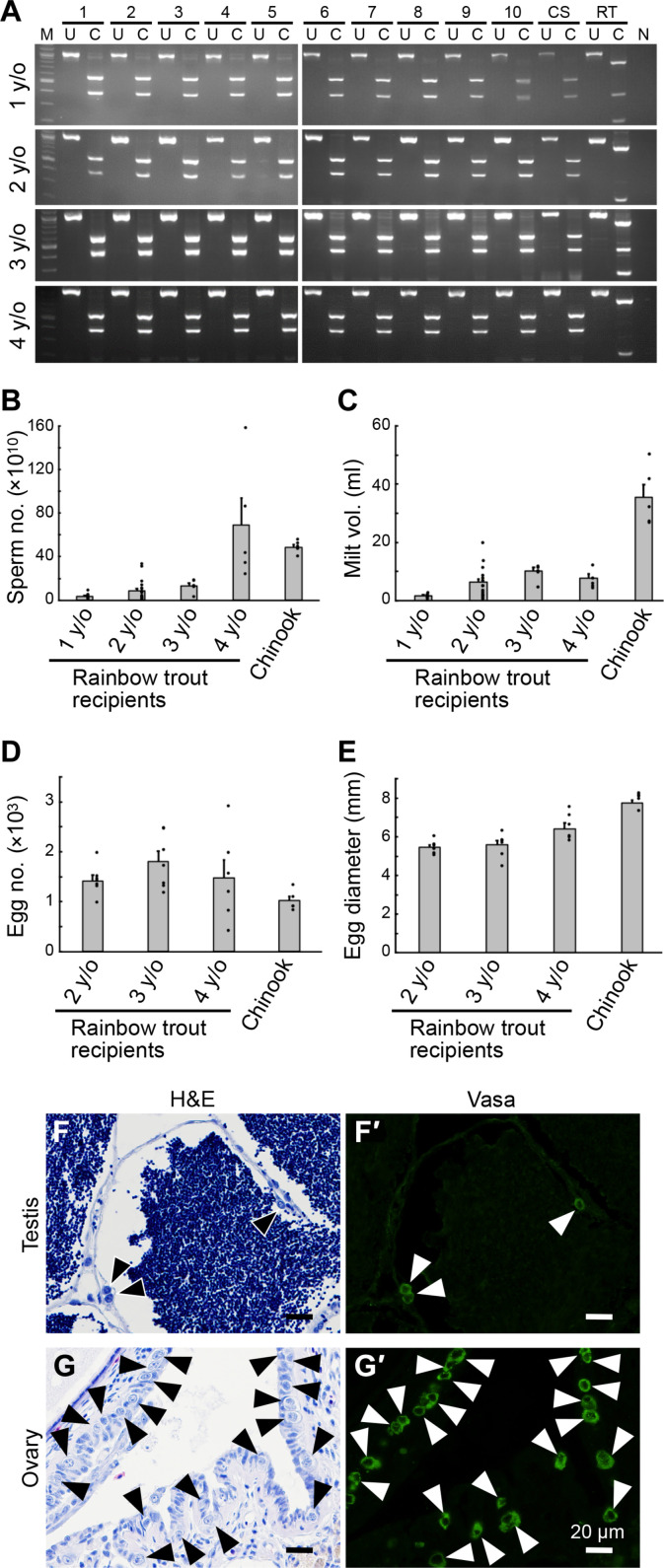
To clarify whether the cell fate of Vasa-positive undifferentiated germ cells is regulated cell autonomously or by the cellular microenvironment, ASGs of Chinook and Kokanee salmon were retrieved from prepubertal individuals and transplanted into the abdominal cavity of the larvae of iteroparous rainbow trout recipients immediately after hatching. The sterility of the recipients is important for the efficient production of eggs and sperm derived from donor germ cells in recipient individuals (14, 15). Therefore, rainbow trout recipients used for transplantation of Chinook salmon germ cells were deficient in endogenous germ cells by knocking out the dnd gene (16). The transplanted donor cells migrated to the gonadal anlagen of the recipient larvae, where they were incorporated and initiated gametogenesis. As a result, 10 of the 27 male rainbow trout transplanted with Chinook salmon germ cells produced sperm at 1 year of age (table S1A). Recipient males that matured at the age of 1 year were reared continuously, and all 10 individuals repeatedly produced spermatozoa every year until they reached the age of 4 years. In addition, seven male recipients matured at 2 years of age, and four of these individuals produced spermatozoa annually for 3 years until they reached 4 years of age (table S1A). To determine whether the sperm were derived from donor Chinook salmon, DNA was extracted from semen produced by these 1- to 4-year-old male recipients and subjected to fingerprint analysis. All the sperm-derived DNA showed fingerprints identical to those of donor Chinook salmon (Fig. 2A). The number of Chinook salmon sperm produced by these rainbow trout recipients ranged from 3.4 ± 2.3 (at age 1) to 13.0 ± 6.1 (at age 3) × 1010 fish until they reached age 3 (Fig. 2B), which was comparable to rainbow trout of the same age. Furthermore, at 4 years of age, the number of Chinook salmon sperm produced by the recipients was similar to that of control Chinook salmon that also matured at 4 years of age (Fig. 2B). Milt volume ranged from 1.8 ± 0.8 (at age 1) to 10.2 ± 2.9 (at age 3) ml, which was comparable to rainbow trout of the same age but less than that of Chinook salmon matured at age 4 (35.4 ml) (Fig. 2C). In females, 7 of the 24 recipients formed eggs at age 2 (table S1B). One of the seven mature female recipients died at age 2, but the remaining six produced eggs and ovulated at age 3. In addition, five of these recipients produced eggs for three consecutive years until they were 4 years old (table S1B). Egg numbers produced by female rainbow trout recipients ranged from 1419 ± 296 (at age 2) to 1800 ± 543 (at age 3) (Fig. 2D). The sum of the number of eggs produced by these rainbow trout recipients during age 2 to age 4 was greater than that produced by 4-year-old Chinook salmon (1017 ± 206). Furthermore, egg diameters of 5.5 ± 0.3 (at age 2) to 6.4 ± 0.7 (at age 4) mm were clearly larger than normal rainbow trout eggs (17) and intermediate between those of Chinook salmon and rainbow trout (Fig. 2E).
Fig. 2. Repeated production of donor-derived Chinook salmon gametes by dnd-knockout rainbow trout recipients.
(A) Restriction fragment length polymorphism (RFLP) analysis of the vasa gene using sperm DNA obtained from 1- to 4-year-old (y/o) trout recipients. Numbers 1 to 10 are sperm samples from trout recipients, CS is Chinook salmon sperm, RT is rainbow trout sperm, U is the uncleaved product of the vasa gene, and C is the restriction enzyme–cleaved product. N indicates negative control without adding templated DNA; M indicates DNA-size marker. (B) Number of sperm produced by 1- to 4-year-old trout recipients. (C) Volume of milt produced by 1- to 4-year-old trout recipients. (D) Number of eggs produced by 2- to 4-year-old trout recipients. (E) Diameter of eggs produced by 2- to 4-year-old trout recipients. Chinook in (B) to (E) indicates number of sperm, milt volume, number of eggs, and egg diameter produced by 4-year-old Chinook salmon, respectively. (F and G) Behavior of donor-derived Chinook salmon germ cells in the gonads of 4-year-old trout recipients was analyzed through H&E staining [(F) and (G)] and immunostaining using the Vasa antibody [(F′) and (G′)]. (F) testis; (G) ovary. Arrowheads indicate spermatogonia [(F) and (F′)] and oogonia [(G) and (G′)]. Scale bars, 20 mm.
The rearing experiment of the recipient individuals was terminated when they reached the age of 4, and the gonads of these individuals were sampled for histological observation. Our previous study confirmed that recipient rainbow trout are completely devoid of endogenous germ cells due to the knockout of dnd (16). Our analysis confirmed that Vasa-positive ASGs remained in the testes of recipients even after the reproductive season at age 4 (Fig. 2, F and F′). Furthermore, many Vasa-positive oogonia remained in the ovaries of female recipients after ovulation (Fig. 2, G and G′). These results strongly suggest that these recipients continuously have the ability to form gametes in subsequent spawning seasons.
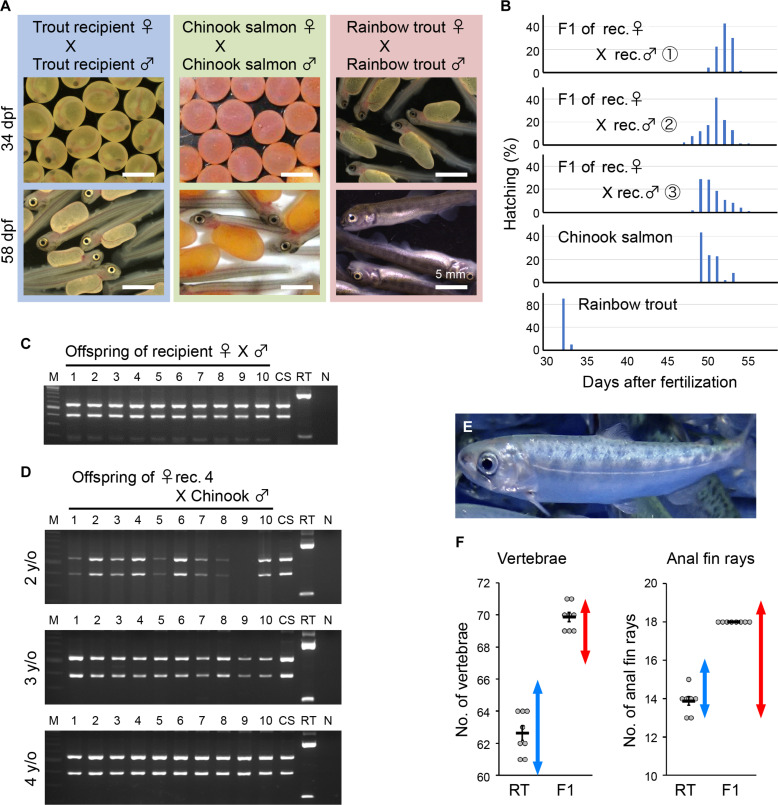
Because it was difficult to extract DNA for fingerprint analysis from the eggs produced by rainbow trout recipients due to the large size and high yolk contents of the eggs, a mating experiment was conducted to obtain offspring using male and female rainbow trout recipients (Fig. 3, A to C, E, and F). As a result, the offspring hatched normally at a similar timing as that of the control Chinook salmon, which was much later than the hatching timing of normal rainbow trout (Fig. 3, A and B). DNA fingerprint analysis of these offspring revealed that fingerprint patterns were identical to those of Chinook salmon (Fig. 3C). The eggs produced by rainbow trout recipients at age 2 to 4 years were artificially inseminated with normal Chinook salmon sperm, and all eggs produced by these recipients were confirmed to be of Chinook salmon origin through DNA fingerprinting (Fig. 3D). The external morphology and growth of the offspring were normal (Fig. 3E), and the number of vertebrae and the number of anal fin rays were within the normal range for Chinook salmon and were clearly different from the recipient rainbow trout (Fig. 3F).
Fig. 3. Production of Chinook salmon offspring by mating dnd-knockout rainbow trout recipients.
(A) Fertilized eggs and hatchlings produced by crosses between male and female trout recipients. The blue, green, and red boxes indicate the offspring of trout recipients, Chinook salmon, and rainbow trout, respectively. The top and bottom panels show offspring at 34 days postfertilization (dpf) and 58 dpf, respectively. (B) Hatching time of the offspring produced by trout recipients. The three randomly selected broods were used for analysis. (C) RFLP analysis of the vasa gene using DNA from the offspring produced by trout recipient parents. Numbers 1 to 10, F1 larvae samples produced by trout recipients; CS, Chinook salmon; RT, rainbow trout. NC indicates the negative control, and M indicates DNA-size marker. (D) RFLP analysis of the vasa gene using DNA from the F1 using eggs from female recipient no. 4, aged 2 to 4 years, and sperm from Chinook salmon. Numbers 1 to 10 represent F1 larvae samples of recipient no. 4. (E) External morphology of the offspring derived from trout recipients. (F) Number of vertebrae and anal fin rays of the offspring produced by trout recipients. RT, values for control rainbow trout; F1, values for the offspring produced by the recipients. Red and blue double-headed arrows indicate the range of respective values for Chinook salmon and rainbow trout from the literature, respectively.
Behavior of Kokanee salmon germ cells in trout recipients
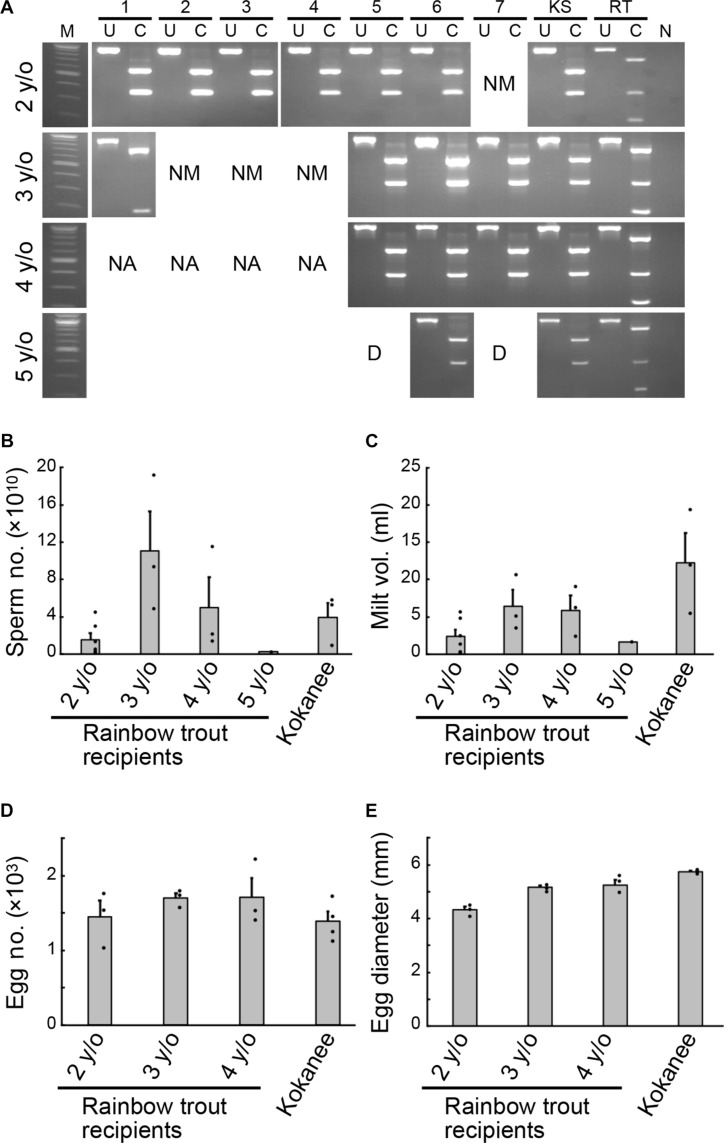
Chinook salmon rarely survive after maturity, and a few precocious males continue spermatogenesis in the following year. Therefore, we conducted a similar experiment using Kokanee salmon, in which all precocious males die (7). In this experiment, infertile rainbow trout recipients produced by triploidization (17, 18) were used as transplantation recipients of Kokanee salmon germ cells. When these recipients reached the age of 2, 6 of the 13 males produced sperm (table S2A). DNA fingerprint analysis revealed that all of these sperm were derived from donor Kokanee salmon (Fig. 4A). Continued rearing of these six individuals and those that did not mature at 2 years of age resulted in three of these recipients producing sperm at 3 years of age (Fig. 4A, nos. 5, 6, and 7). Similarly, when these recipients were reared continuously, all three produced sperm of Kokanee salmon origin at the age of 4 years. Notably, one of them produced sperm of Kokanee salmon origin at the age of 5 years. This individual (no. 6) produced sperm from the Kokanee salmon continuously for 4 years (Fig. 4A). In addition, two of the remaining six individuals (Fig. 4A, nos. 5 and 7) repeatedly produced sperm for at least 2 years. The number of sperm produced by these recipients was 0.15 (at age 5) to 11.1 ± 0.7 (at age 3) × 1010 (Fig. 4B), and the semen volume was 1.7 (at age 5) to 6.4 ± 3.7 (at age 3) ml (Fig. 4C). These values were within the normal range for rainbow trout sperm.
Fig. 4. Repeated production of donor-derived Kokanee salmon gametes by dnd-knockout rainbow trout recipients.
(A) RFLP analysis of the vasa gene using sperm DNA obtained from 2- to 5-year-old trout recipients. Numbers 1 to 7 are sperm samples from trout recipients, KS is Kokanee salmon sperm, RT is rainbow trout sperm, U is the uncleaved product of the vasa gene, and C is the restriction enzyme-cleaved product. N indicates negative control in which PCR was performed without adding template DNA; M indicates DNA-size marker. NM indicates not mature; NA indicates not analyzed; D indicates dead. (B) Number of sperm produced by 2- to 5-year-old trout recipients. Kokanee indicates the number of sperm produced by 3-year-old Kokanee salmon. (C) Volume of milt produced by 2- to 5-year-old trout recipients; Kokanee indicates the milt volume produced by 3-year-old Kokanee salmon. (D) Number of eggs produced by 2- to 4-year-old trout recipients. Kokanee indicates the number of eggs produced by a 3-year-old Kokanee salmon. (E) Average diameter of eggs produced by 2- to 4-year-old trout recipients. Kokanee shows the diameter of eggs produced by 3-year-old Kokanee salmon.
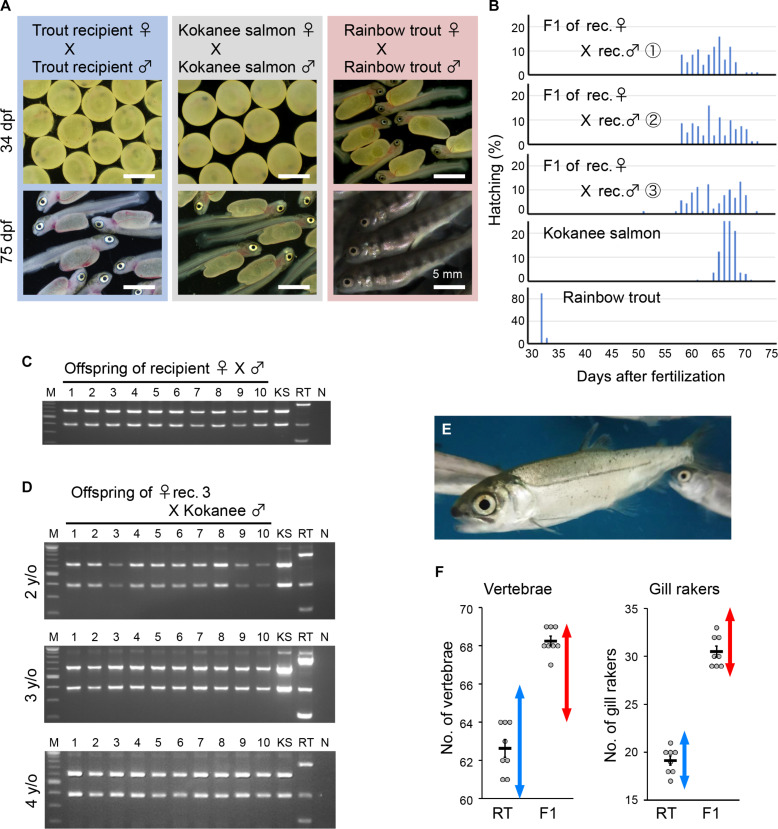
In female recipients transplanted with Kokanee salmon germ cells, recipient no. 3, repeatedly produced eggs from 2 to 4 years of age. In addition, recipient no. 4 repeatedly produced eggs three times from 3 to 5 years of age (table S2B). The number of eggs produced by these recipients was comparable to the number of eggs produced by 3-year-old Kokanee salmon each year (Fig. 4D). Egg size tended to increase with the recipient’s age (Fig. 4E). To determine whether the eggs produced by these rainbow trout recipients derived from the donor Kokanee salmon, a mating experiment was conducted using the male and female recipients (Fig. 5, A to C, E, and F). As a result, the hatching timing of the resulting offspring was markedly later than that of rainbow trout and similar to that of Kokanee salmon (Fig. 5, A and B). The offspring from the rainbow trout recipient parents were subjected to DNA fingerprint analysis, and all of them showed the same pattern as that of the Kokanee salmon (Fig. 5C). The eggs produced by rainbow trout recipients at the age of 2 to 4 years were artificially inseminated with normal Kokanee salmon sperm to produce the next generation. All the offspring were identified as pure Kokanee salmon through DNA fingerprinting (Fig. 5D). The offspring showed normal growth and external morphology (Fig. 5E), and the vertebral numbers and gill raker numbers of these individuals were consistent with those of Kokanee salmon and clearly distinguishable from those of rainbow trout (Fig. 5F).
Fig. 5. Production of Kokanee salmon offspring by mating triploid rainbow trout recipients.
(A) Fertilized eggs and hatchlings produced by crosses between male and female trout recipients. The blue, gray, and red boxes indicate the offspring of trout recipients, Kokanee salmon, and rainbow trout, respectively. The top and bottom panels show offspring at 34 dpf and 75 dpf, respectively. (B) Hatching time of the offspring produced by trout recipients. The three broods produced by 3-year-old recipients were used for analysis. (C) RFLP analysis of the vasa gene using DNA of the offspring produced by trout recipient parents. Numbers 1 to 10, F1 larvae produced by trout recipients; KS, Kokanee salmon; RT, rainbow trout. NC indicates the negative control, and M indicates Mw marker. (D) RFLP analysis of the vasa gene using DNA from the offspring produced using eggs from female recipient no. 3, aged 2 to 4 years, and sperm from Kokanee salmon. Numbers 1 to 10 represent F1 larvae samples from recipient no. 3. (E) External morphology of the offspring produced by trout recipients. (F) Number of vertebrae and gill rakers of the offspring produced by trout recipients. RT, values for control rainbow trout; F1, values for the offspring produced by the recipients. Red and blue double-headed arrows indicate the range of respective values for Kokanee salmon and rainbow trout from the literature, respectively.
DISCUSSION
In this study, we found that semelparous salmon completely lost undifferentiated germ cells that were expected to contain germline stem cells after first sexual maturation in both sexes. On the other hand, stem cells were maintained in iteroparous rainbow trout after the reproductive seasons and prepared for gametogenesis in the following reproductive season. To investigate the cause of this difference in the behavior of germline stem cells, we transplanted semelparous salmon germ cells into iteroparous rainbow trout recipients and found that the male recipients continued to produce donor-derived salmon gametes for at least 4 years and the females continued to produce the gametes for at least 3 years. dnd-knockout rainbow trout recipients that received Chinook salmon germ cells survived beyond the 4-year-old spawning season, and many undifferentiated germ cells derived from the donor Chinook salmon were detected in the gonads of the postmaturation recipients. This suggests that these recipients are most likely to reproduce during the fifth reproductive season. Overall, these facts indicate that the somatic environment surrounding germline stem cells has a major influence on cell fate if undifferentiated germ cells (presumably germline stem cells) survive in the gonads after each reproductive season. Triploids were used as the rainbow trout recipients to which Kokanee salmon germ cells were transplanted. Thus, the rainbow trout’s own undifferentiated germ cells were present in their gonads (19), causing difficulties in distinguishing between recipient-derived and donor-derived germ cells. However, the fact that triploid rainbow trout repeatedly produced Kokanee salmon gametes suggests that the fate determination of undifferentiated germ cells in Kokanee salmon is also likely to be controlled by their cellular environments. Although the mechanism by which undifferentiated germ cells are lost in semelparous salmon is still not clear, the germ cell transplantation system established in this study between semelparous and iteroparous species can provide a powerful tool to study the regulatory mechanisms of germ cell differentiation, survival, and proliferation.
Another fact revealed by this germ cell transplantation study was that the timing of the onset of maturation, or puberty, is not germ cell–autonomous but is determined by the somatic environments surrounding the germ cells. Although Chinook salmon generally require 3 to 7 years to reach maturity and at least 2 years for some precocious males (20), many rainbow trout recipients produce Chinook salmon sperm annually from the age of 1 year and their eggs annually from the age of 2 years. Although it is difficult to conclude because few precociously mature Kokanee salmon mature at the same age as rainbow trout (7), the results of this study indicate that, similar to Chinook salmon, the maturation of recipient rainbow trout may occur earlier than in normal Kokanee salmon. These facts indicate that the cellular environment is more important for puberty initiation in salmonids than germ cell–autonomous factors.
The numbers of Chinook salmon sperm produced by rainbow trout recipients were lower than those of 4-year-old Chinook salmon until the recipients reached age 3, but this was likely dependent on the recipient’s body size. Sperm numbers produced by 4-year-old recipients were comparable to those of Chinook salmon. The numbers of Kokanee salmon sperm produced by 2-year-old rainbow trout recipients were also lower than those produced by control Kokanee salmon because control Kokanee salmon mature at age 3. The 3-year-old recipients produced sperm comparable to those in the control. A trend toward lower semen volume was observed in rainbow trout recipients in the current study, although the reason for this could not be explained. However, these semen volumes were within the normal range of rainbow trout semen volume (17), suggesting that the amount of semen is probably dependent on the recipient species rather than the donor species.
Both Chinook and Kokanee salmon produced larger eggs than normal rainbow trout (~4.8 mm) (17); however, eggs from donor species produced by rainbow trout recipients were found to be larger than those of rainbow trout. This indicates that egg size is at least partially influenced by the donor germ cells. Similarly, donor species have been reported to affect the size of eggs produced by recipients in the family Cyprinidae (21), and this phenomenon may be general and not limited to salmonids. The number of eggs produced by these recipients tended to be fewer than that produced by normal rainbow trout, but this may be due to compensation for the larger egg sizes.
Eggs produced by rainbow trout recipients receiving Kokanee salmon germ cells varied greatly in quality. This was most likely a donor issue, as the survival rate of Kokanee salmon in the control was also not high. The population used in this experiment has been reproduced repeatedly as a genetically small population for a long time, which may have resulted in inbreeding. In general, fish eggs decrease in developmental ability with time after ovulation, a phenomenon called overripening. Kokanee salmon eggs overripen much faster than those of rainbow trout, so we cannot rule out the possibility that the problem of overriping has become apparent.
We revealed distinct functional differences in the gonads of semelparous and iteroparous salmon. The differences between semelparous and iteroparous salmon were not only dying after their first reproduction but also the behavior of their germline stem cells after their first gametogenesis. Although the molecular mechanisms regulating this germ cell behavior are not known, somatic environments surrounding the germ cells play a major role in regulating this germ cell behavior. Various omics studies using the fish species used in this study are expected to elucidate these molecular mechanisms as well. This is not only a biologically important discovery but is also expected to contribute to the enhancement of Pacific salmon seedling production technology.
MATERIALS AND METHODS
Fish
Twenty-five male Chinook salmon were used as donors for germ cell transplant experiments (body weight, 132.6 ± 2.1 g), which were provided by the National Oceanic and Atmospheric Administration Northwest Fisheries Science Center. In the Kokanee salmon experiment, 10 males (body weight, 71.3 ± 9.3 g) that had been passaged in spring water at 10.5°C at the Oizumi Station (Yamanashi, Japan) of the Tokyo University of Marine Science and Technology were used as donors. The donor testes of both species used in this study retained only ASGs but not more differentiated germ cells.
For the Chinook salmon germ cell transplantation, rainbow trout hatchlings produced by mating dnd heterozygous mutant females and males were used as recipients. Of the resulting hatchlings, genotyping with T7 endonuclease using DNA prepared from their mucous was performed using the protocol of Fujihara et al. (16), leaving only homozygous mutants for subsequent transplantation experiments. When transplanting Kokanee salmon germ cells, triploid rainbow trout were used as recipients. To produce triploid recipients, fertilized rainbow trout eggs were raised in 10°C water for 10 min after fertilization and immersed in 27°C water for the next 15 min and then allowed to develop and grow in environmental water at 10.5°C. All procedures described herein were approved by Tokyo University of Marine Science and Technology, Institutional Animal Care and Use Committee (no. 125-S5).
Germ cell transplantation
After anesthetizing the donor individuals, the abdomen was incised, and the testes were removed. The testicular mesentery and accompanying blood vessels were removed from the testes using tweezers under a stereomicroscope. The testes were also cut into small pieces using Weckel scissors (MB-41, NAPOX, Natsume Seisakusho Co., Ltd., Tokyo, Japan) to obtain testis fragments. The resulting testis fragments were transferred into 1 ml of trypsin (150 U/mgP, 88% protein, Worthigton Biochemical Corp., New Jersey, USA)/phosphate-buffered saline (+) solution and subjected to enzymatic digestion for 2 hours at 20°C. During the enzymatic treatment, gentle pipetting was applied every 30 min to promote the dissociation of remaining testicular fragments. The resulting testicular cell suspension was filtered through a nylon screen with 42-μm mesh to remove cell clumps resulting from incomplete dissociation. The resulting donor testicular cells were stored at 4°C until transplantation.
Glass micropipettes for germ cell transplantation were prepared by using a glass capillary (G-1, Narishige Scientific Instruments Laboratory, Tokyo, Japan) with a puller (PW-6, Narishige Scientific Instruments Laboratory). The inner diameter of the tip of the glass micropipette was adjusted to 70 to 90 μm using a microgrinder (EG-3, Narishige Scientific Instruments Laboratory). A micromanipulator (BP-1, Narishige Scientific Instruments Laboratory) and microinjector (IM-9A, Narishige Scientific Instruments Laboratory) set up under a stereomicroscope were used to transplant 20 to 30 nl of the donor cell suspension containing 20,000 to 30,000 testicular cells into the abdominal cavity of the newly hatched recipient larvae (22, 23). The resulting recipients were then reared in running water at 10.5°C.
Histology
Immature testes were isolated from 8-month-old rainbow trout [body weight, 12.5 g; gonadosomatic index (GSI), 0.04%], 15-month-old Chinook salmon (body weight, 26.8 g; GSI, 0.13%), and 7-month-old Kokanee salmon (body weight, 3.2 g; GSI, 0.02%). Immature ovaries were isolated from 8-month-old rainbow trout (body weight, 13.2 g; GSI, 0.08%), 15-month-old Chinook salmon (body weight, 26.8 g; GSI, 0.14%), and 7-month-old Kokanee salmon (body weight, 5.0 g; GSI, 0.15%). Mature testes were isolated from 2-year-old rainbow trout (body weight, 561 g; GSI, 5.2), 4-year-old Chinook salmon (body weight, 1151 g; GSI, 6.27), and 3-year-old Kokanee salmon (body weight, 806 g; GSI, 2.64). Postovulatory ovaries were isolated from 3-year-old rainbow trout (body weight, 1571 g; GSI, 0.46), 4-year-old Chinook salmon (body weight, 1343 g; GSI, 0.71%), and 3-year-old Kokanee salmon (body weight, 868 g; GSI, 0.78%). Mature testes and postovulatory ovaries of dnd-knockout rainbow trout recipients transplanted with Chinook salmon germ cells were both isolated from 4-year-old fish (male body weight, 1117 g; GSI, 3.4%; female body weight, 1274 g; GSI, 3.4%). These tissues were cut into 5-mm cubes, fixed with Bouin’s solution, cut into 4-μm-thick sections, and subjected to immunohistochemical staining and hematoxylin and eosin staining. Immunohistochemical staining was performed according to the method of Nakajima et al. (24), and a primary antibody against zebrafish Vasa (ab209710, Abcam, Cambridge, UK) was used.
Analyses of gametes obtained from recipients
Gamete production was determined by applying gentle pressure to the abdomen of the recipients. This operation was performed once a week during the spawning season. If sperm were obtained, then the semen was squeezed, and its total volume and sperm count were quantified. This analysis was performed on all spermiated recipients, and the values are expressed as the means (± SE). Restriction fragment length polymorphism analysis using sperm DNA was performed to confirm whether the sperm obtained from the male recipients were of donor origin. Total DNA was extracted from 1 μl of milt with a Gentra Puregene Tissue Kit (QIAGEN, Venlo, The Netherlands) according to the attached protocol. Primers conserved among rainbow trout, Chinook salmon, and Kokanee salmon (forward: 5′-GACCTCTCATGCAAACGCTTCA-3′/reverse: 5′-CAGACCCATACCTTCCTGCTATCA-3′) were used to amplify a partial fragment of the vasa gene. Cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 60 s were repeated 35 times using the method described by Hamasaki et al. (25). The amplified vasa fragments were digested with Mbo I (Takara Bio Inc.) to distinguish between recipient rainbow trout-derived sequences and those from donor Chinook salmon or Kokanee salmon. If eggs were obtained from female recipients, then the number of eggs and egg diameter were quantified. All ovulated individuals were used in this analysis, and the results are expressed as the means (± SE).
Mating study
Host-derived gametes were subjected to fertilization by using the isotonic method. The eggs were reared in running water at 10.5°C, and the fertilization rate, eyed egg rate, hatching rate, and swim-up rate were determined. Eggs obtained from some recipients were also used for fertilization tests using sperm from control Chinook or Kokanee salmon. For the next-generation larvae produced using male and female rainbow trout recipients, hatching dates were recorded, and these values were compared with the hatching timings of Chinook salmon, Kokanee salmon, and rainbow trout. DNA obtained from the resulting swim-up fry was prepared using the method described above, and species identification was performed in the same manner as for sperm analysis.
Recipients that produced donor-derived gametes in the Chinook or Kokanee salmon germ cell transplantation experiments were reared continuously until they reached 4 or 5 years old, respectively. These recipients were checked annually for gamete production, and if eggs or sperm were obtained, then fertilization was performed using these recipients though the method described above.
The next generation obtained by fertilizing eggs and sperm collected from the females and males of 3-year-old recipients was kept in running water at 10.5°C, and morphological analyses were conducted at the age of 1 year. The number of vertebrae and the number of fin rays on the anal fin were counted to discriminate between rainbow trout and Chinook salmon. In contrast, the number of vertebrae and gill rakers were counted to discriminate between rainbow trout and Kokanee salmon.
Acknowledgments
We are grateful to P. Swanson (NOAA) for providing testes of Chinook salmon used in this study.
Funding: This work was supported by the Ministry of Education, Culture, Sports, Science and Technology, Japan, 23H00344 (G.Y.); and by the Japan Science and Technology Agency, JPMJMI21C1 (G.Y.).
Author contributions: Conceptualization: G.Y. Methodology: G.Y., R.F., S.N., K.Kan., K.Kam., M.T., N.M., M.M., M.H., and S.S. Investigation: G.Y., R.F., S.N., K.Kan., K.Kam., M.T., N.M., M.M., M.H., and S.S. Resources: G.Y., R.F., S.N., K.Kan., K.Kam., M.T., N.M., M.M., M.H., and S.S. Validation: G.Y., R.F., S.N., K.Kan., K.Kam., M.T., N.M., M.M., M.H., and S.S. Formal analysis: G.Y., R.F., S.N., K.Kan., K.Kam., M.T., N.M., M.M., M.H., and S.S. Visualization: G.Y., R.F., S.N., K.Kan., K.Kam., M.T., N.M., M.M., M.H., and S.S. Funding acquisition: G.Y. Project administration: G.Y. Supervision: G.Y. Writing—original draft: G.Y. Writing—review and editing: R.F., S.N., K.Kan., K.Kam., M.T., N.M., M.M., M.H., and S.S.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Tables S1 and S2
REFERENCES AND NOTES
- 1.C. Groot, L. Margolis, Pacific Salmon Life Histories (UBC Press, 1991). [Google Scholar]
- 2.Ishida R., Takagi K., Arita S., Criteria for the differentiation of mature and immature forms of chum and sockeye salmon in northern seas. Int. North. Pac. Fish. Commi. Bull. 5, 27–47 (1961). [Google Scholar]
- 3.Weisel G. F., A histological study of the testes of the sockeye salmon (Oncorhynchus nerka). J. Morphol. 73, 207–229 (1943). [Google Scholar]
- 4.Hiroi O., Yamamoto K., Studies on the maturation of salmonid fishes-1: Changes in the testis of the chum salmon, Oncorhynchus keta, during anadromous migration. Bull. Fac. Fish. Hokkaido Univ. 19, 173–184 (1968). [Google Scholar]
- 5.R. J. Behnke, Native Trout of Western North America (American Fisheries Society, 1992). [Google Scholar]
- 6.Narum S. R., Hatch D., Talbot A. J., Moran P., Powell M. S., Iteroparity in complex mating systems of steelhead Oncorhynchus mykiss (Walbaum). J. Fish Biol. 72, 45–60 (2008). [Google Scholar]
- 7.R. L. Burgner, “Life history of sockeye salmon (Oncorhynchus nerka)” in Pacific Salmon Life Histories, C. Groot, L. Margolis, Eds. (UBC Press, 1991), pp. 1–118. [Google Scholar]
- 8.Van den Hurk R., Peute J., Vermeij J., Morphological and enzyme cytochemical aspects of the testis and vas deferens of the rainbow trout, Salmo gairdneri. Cell Tissue Res. 186, 309–325 (1978). [DOI] [PubMed] [Google Scholar]
- 9.Sumpter J. P., Scott A. P., Baynes S. M., Witthames P. R., Early stages of the reproductive-cycle in virgin female rainbow-trout (Salmo gairdneri Richardson). Aquaculture 43, 235–242 (1984). [Google Scholar]
- 10.Billard R., Reproduction in rainbow trout: Sex differentiation, dynamics of gametogenesis, biology and preservation of gametes. Aquaculture 100, 263–298 (1992). [Google Scholar]
- 11.Zelennikov O., Comparative analysis of the state of ovaries in juvenile Pacific salmons as related to the problem of monocyclicity formation. J. Ichthyology 43, 445–453 (2003). [Google Scholar]
- 12.Yano A., Suzuki K., Yoshizaki G., Flow-cytometric isolation of testicular germ cells from rainbow trout (Oncorhynchus mykiss) carrying the green fluorescent protein gene driven by trout vasa regulatory regions. Biol. Reprod. 78, 151–158 (2008). [DOI] [PubMed] [Google Scholar]
- 13.Yoshizaki G., Ichikawa M., Hayashi M., Iwasaki Y., Miwa M., Shikina S., Okutsu T., Sexual plasticity of ovarian germ cells in rainbow trout. Development 137, 1227–1230 (2010). [DOI] [PubMed] [Google Scholar]
- 14.Yoshizaki G., Takashiba K., Shimamori S., Fujinuma K., Shikina S., Okutsu T., Kume S., Hayashi M., Production of germ cell-deficient salmonids by dead end gene knockdown, and their use as recipients for germ cell transplantation. Mol. Reprod. Dev. 83, 298–311 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Yoshikawa H., Takeuchi Y., Ino Y., Wang J., Iwata G., Kabeya N., Yazawa R., Yoshizaki G., Efficient production of donor-derived gametes from triploid recipients following intra-peritoneal germ cell transplantation into a marine teleost, Nibe croaker (Nibea mitsukurii). Aquaculture 478, 35–47 (2017). [Google Scholar]
- 16.Fujihara R., Katayama N., Sadaie S., Miwa M., Matias G. A. S., Ichida K., Fujii W., Naito K., Hayashi M., Yoshizaki G., Production of germ cell-less rainbow trout by dead end gene knockout and their use as recipients for germ cell transplantation. Mar. Biotechnol. 24, 417–429 (2022). [DOI] [PubMed] [Google Scholar]
- 17.Lee S., Iwasaki Y., Shikina S., Yoshizaki G., Generation of functional eggs and sperm from cryopreserved whole testes. Proc. Natl. Acad. Sci. U.S.A. 110, 1640–1645 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okutsu T., Shikina S., Kanno M., Takeuchi Y., Yoshizaki G., Production of trout offspring from triploid salmon parents. Science 317, 1517 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Carrasco L. A. P., Doroshov S., Penman D. J., Bromage N., Long-term, quantitative analysis of gametogenesis in autotriploid rainbow trout, Oncorhynchus mykiss. J. Reprod. Fertil. 113, 197–210 (1998). [DOI] [PubMed] [Google Scholar]
- 20.M. Healey, “Life history of Chinook salmon (Oncorhynchus nerka)” in Pacific Salmon Life Histories, C. Groot, L. Margolis, Eds. (UBC Press, 1991), pp. 311–394. [Google Scholar]
- 21.Franek R., Kaspar V., Shah M. A., Gela D., Psenicka M., Production of common carp donor-derived offspring from goldfish surrogate broodstock. Aquaculture 534, 736252 (2021). [Google Scholar]
- 22.Takeuchi Y., Yoshizaki G., Takeuchi T., Generation of live fry from intraperitoneally transplanted primordial germ cells in rainbow trout. Biol. Reprod. 69, 1142–1149 (2003). [DOI] [PubMed] [Google Scholar]
- 23.Okutsu T., Suzuki K., Takeuchi Y., Takeuchi T., Yoshizaki G., Testicular germ cells can colonize sexually undifferentiated embryonic gonad and produce functional eggs in fish. Proc. Natl. Acad. Sci. U.S.A. 103, 2725–2729 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakajima S., Hayashi M., Kouguchi T., Yamaguchi K., Miwa M., Yoshizaki G., Expression patterns of gdnf and gfrα1 in rainbow trout testis. Gene Expr. Patterns 14, 111–120 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Hamasaki M., Takeuchi Y., Yazawa R., Yoshikawa S., Kadomura K., Yamada T., Miyaki K., Kikuchi K., Yoshizaki G., Production of tiger puffer Takifugu rubripes offspring from triploid grass puffer Takifugu niphobles parents. Mar. Biotechnol. 19, 579–591 (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1 and S2