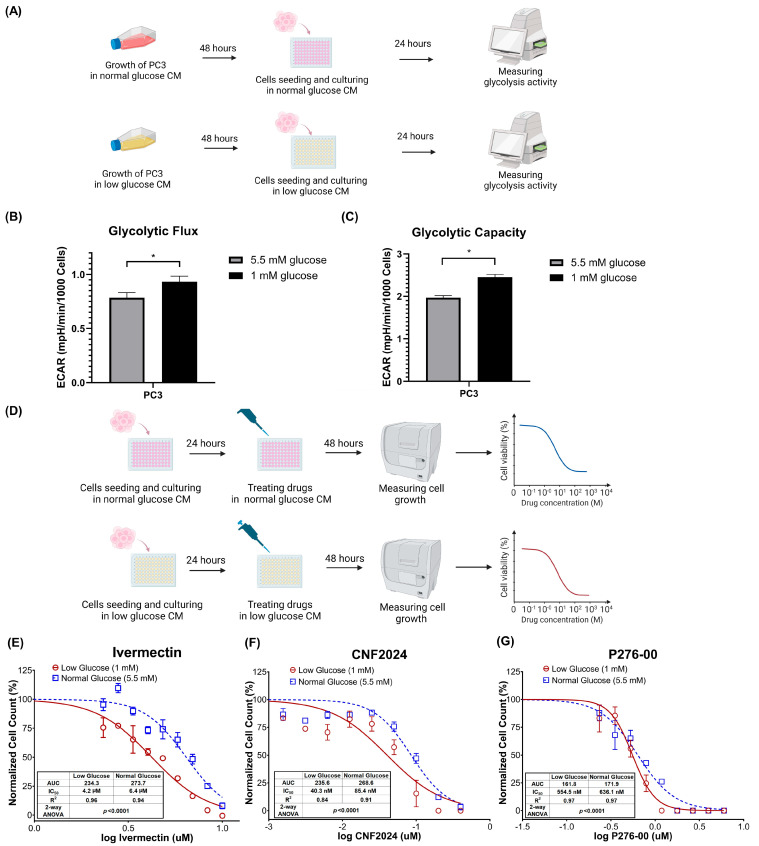
Figure 4.
Experimental validation of candidate drugs in PC3 models with normal- and high-glycolysis conditions. (A) Workflow of development of glycolytic PC3 cells and stress test assays. (B) Glycolytic flux and (C) glycolytic capacity of PC3 after culturing in conditional media (CM) for 72 h. * p < 0.05, Student’s t-test. Representative results of three independent experiments with 8 to 10 replicates per group. (D) Workflow of drug sensitivity evaluation. (E–G) Dose–response curve of PC3 after ivermectin, CNF2024, and P276-00 treatment for 48 h, respectively, in either normal-glucose or low-glucose media. There were 3 replicates per group, and the experiments were repeated 3 times. The statistical analysis was performed using two-way ANOVA. Abbreviations: CM: conditional media; ECAR: extracellular acidification rate.