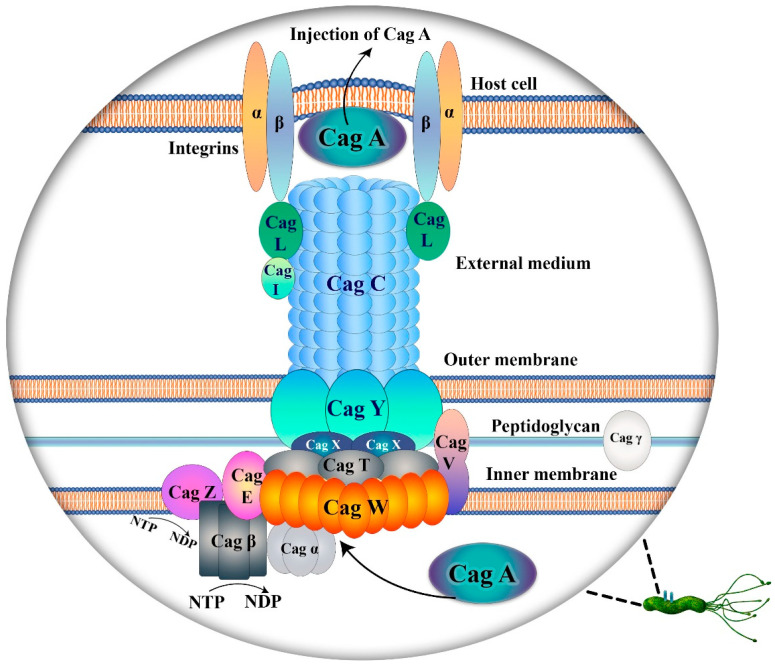
Figure 1.
Helicobacter pylori type IV secretion system (T4SS) and Cag A pathogenicity. In the intricate interplay between H. pylori adhesins and epithelial cells, various receptors play a crucial role in mediating binding. A noteworthy homology has been observed between H. pylori outer membrane proteins and Vir proteins in Agrobacterium., The cagA pathogenicity island consists of distinct elements within the multicomponent T4SS complex. Specifically, Cag X, T, and Y contribute to the core complex, while CagE, W, and V participate in the inner membrane complex. Additionally, CagC, L, and I are instrumental in pilus formation. Subsequent to the interaction between host cells and binding proteins, the CagA substrate is delivered through assembled pili. Integrin receptors play a pivotal role in facilitating this interaction with CagA, Y, L, and I. In the lower section of the diagram, CagE, Z, α, and β are implicated in generating energy through dephosphorylation of nucleoside triphosphates (NTP), ultimately leading to translocation of CagA. Notably, Cagγ, situated in the peptidoglycan layer, assumes responsibility for hydrolyzing peptidoglycan.