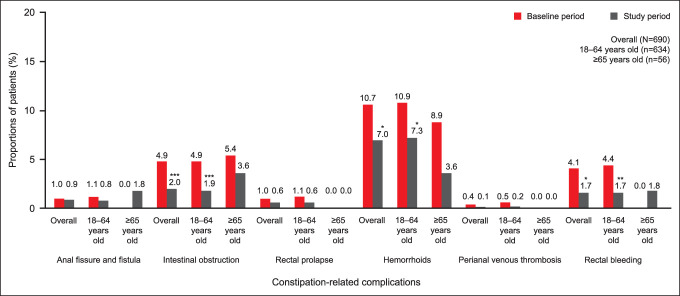
Figure 3.
Constipation-related complications 6 months before prucalopride initiation (baseline period) and 6 months after prucalopride initiation (study period) in patients with CIC. All constipation-related complications for which at least 1 occurrence was recorded in all patients; no occurrence of perianal/perirectal abscess was recorded. P values for the change in constipation-related complications from baseline to 6 months after prucalopride initiation were calculated using McNemar tests. *P < 0.05, **P < 0.01, ***P < 0.001. CIC, chronic idiopathic constipation.