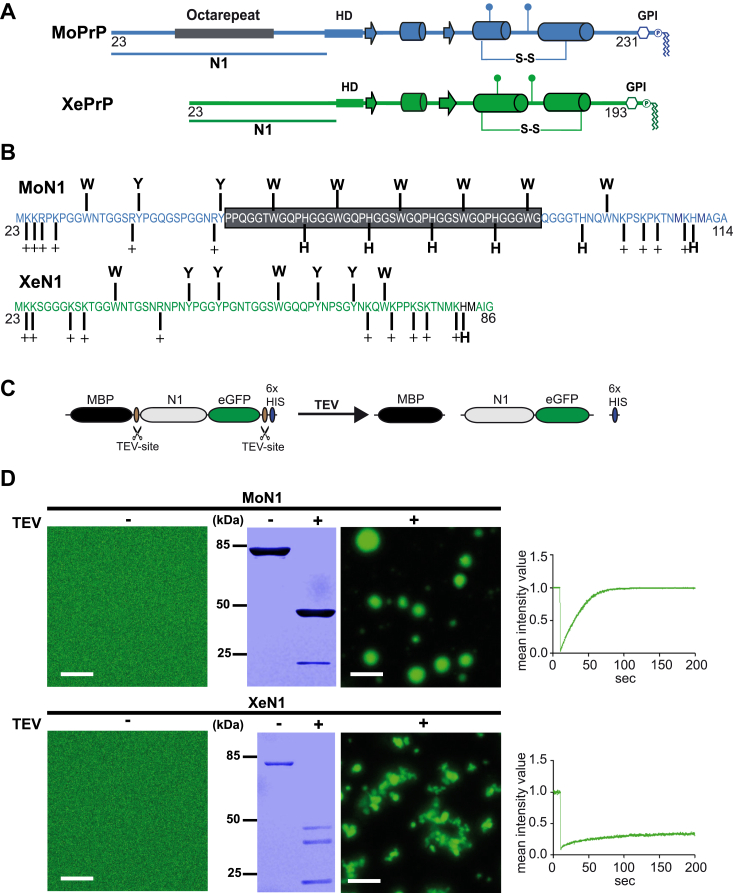
Figure 1.
MoN1 undergoes LLPS, while XeN1 forms undynamic assemblies.A, schematic presentation of MoPrP (blue) and XePrP (green). Mouse octarepeat: gray box; arrows: beta strands; tubes: alpha helices. B, amino acid sequence of MoN1 and XeN1. Aromatic amino acids, positively charged amino acids, and histidines are highlighted. The presence of the histidine in XeN1 is due to the 3F4 epitope that was inserted to allow antibody detection of XePrP. Neither MoN1 nor XeN1 contains amino acids with negatively charged side chains. C, schematic presentation of the experimental approach. After expression in Escherichia coli and purification, phase separation is induced by removing the N-terminal maltose-binding protein (MBP) and C-terminal His tag (6x HIS) by incubation with TEV protease. D, MoN1-GFP and XeN1-GFP (10 μM in 10 mM Tris, pH 7.4) were analyzed by laser scanning microscopy before (TEV-) and after addition of TEV protease (TEV+). TEV protease cleavage was performed for 1 h. The scale bar represents 10 μm (left panels). An aliquot of each sample (4.5 μg) was analyzed in parallel by SDS-PAGE and Coomassie brilliant blue staining (middle panels). Protein mobility within the droplets was measured by fluorescence recovery after photobleaching (FRAP). After 10 s of baseline recording (pre-bleach), a small area of interest (AOI) was photobleached. The average normalized fluorescence intensity of three AOIs was plotted over time (right panels). C-C: disulphide bond; GPI: glycosylphosphatidylinositol anchor; HD, hydrophobic domain; LLPS, liquid-liquid phase separation; TEV, tobacco etch virus.