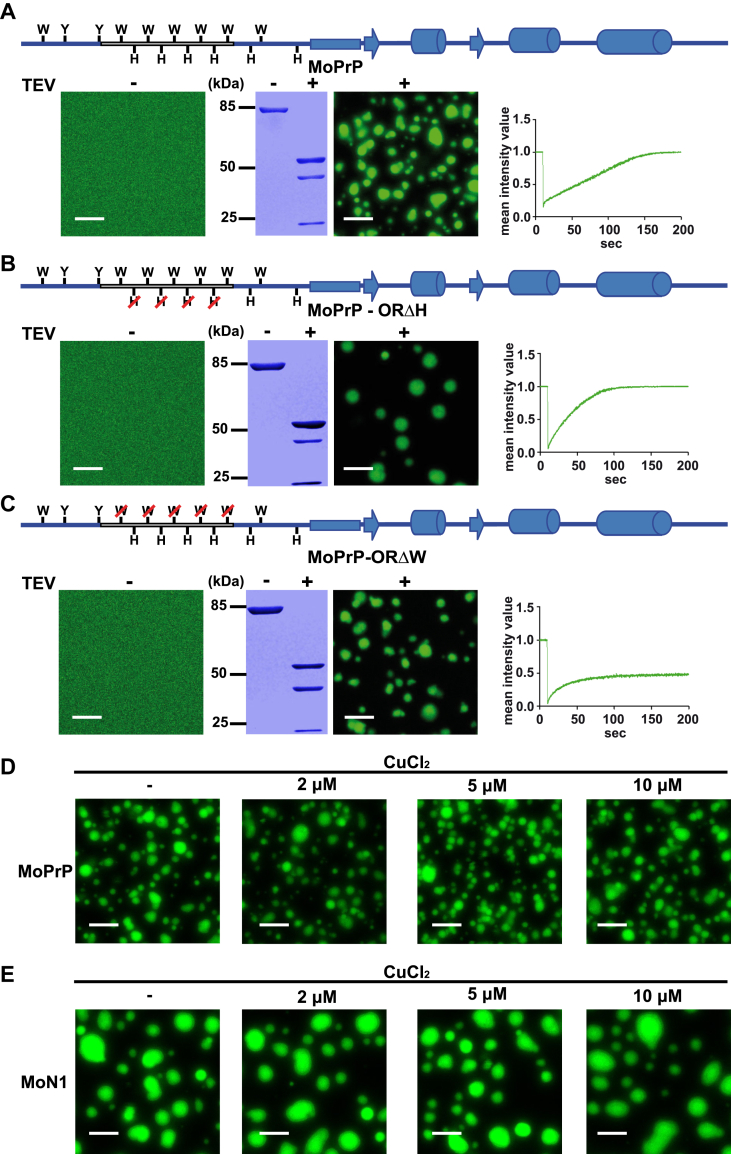
Figure 3.
Liquid-liquid phase separation of full-length PrP is promoted by the tryptophans in the octarepeat and not influenced by copper.A, schematic presentation of WT MoPrP (top panel). Ten µM of MoPrP in 10 mM Tris, pH 7.4 was analyzed by laser scanning microscopy before (TEV-) or after (TEV+) incubation with TEV protease for 1 h. The scale bar represents 10 μm. An aliquot of each sample (4.5 μg) was analyzed in parallel by SDS-PAGE and Coomassie brilliant blue staining (middle panels). Protein mobility within the droplets was measured by fluorescence recovery after photobleaching (FRAP) as described in Figure 1D. B, schematic presentation of MoPrP-ORΔH. The histidine residues in the octarepeat were replaced by glycines. Fluorescence microscopy and FRAP recordings were performed as described in (A). C, schematic presentation of MoPrP-ORΔW. The tryptophanes in the octarepeat were replaced by glycines. Fluorescence microscopy and FRAP recordings were performed as described in (A). D, ten µM of WT MoPrP in 10 mM Tris–HCl, pH 7.4 containing increasing amounts of CuCl2 as indicated was incubated with TEV protease for 1 h and then analyzed by laser scanning microscopy. The scale bar represents 10 μm. E, ten µM of WT MoN1 in 10 mM Tris–HCl, pH 7.4 containing increasing concentrations of CuCl2 as indicated was incubated with TEV protease for 1 h and then analyzed by laser scanning microscopy. The scale bar represents 10 μm. TEV, tobacco etch virus.