Figure 2.
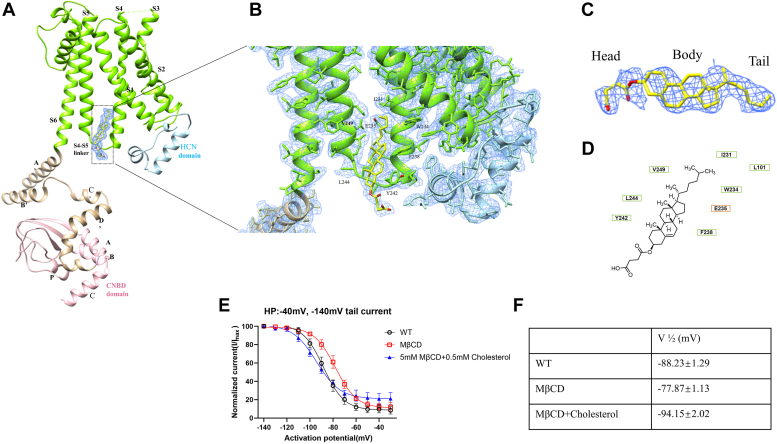
CHS binding site and its effect on HCN3 channel.A, cartoon representation of one subunit of HCN3 channel with individual domains colored. B, CHS-binding pocket. The discernible nonprotein density observed within the intracellular domain of HCN3, created by the terminal regions of S4, S5, and the S4-S5 linker, tightly accommodates CHS (colored as yellow). C, the “head,” “body,” and “tail” of CHS are referred to as the hemisuccinate group, steroid ring, and alkane group, respectively. D, chemical structure of CHS and its interaction with surrounding amino acids. E, steady-state activation curves of WT HCN3, MβCD-treated HCN3, and reintroduced cholesterol-treated HCN3 currents obtained by whole-cell voltage-clamp recordings showed that HCN3 channel is inhibited by cholesterol. Data points are mean ± SEM of three to five cells. F, the voltage of half-maximal activation (V1/2) determined by Boltzmann fit to the mean of steady-state activation curve from Figure 2E. CHS, cholesteryl hemisuccinate; CNBD, cyclic nucleotide-binding domain; HCN, Hyperpolarization-Activated Cyclic Nucleotide-gated; MβCD, methyl-β-cyclodextrin.