Figure 3.
CB1R-mediated reduction of glutamate release is independent of the inhibition of voltage-gated Ca2+ channels in RBC terminals
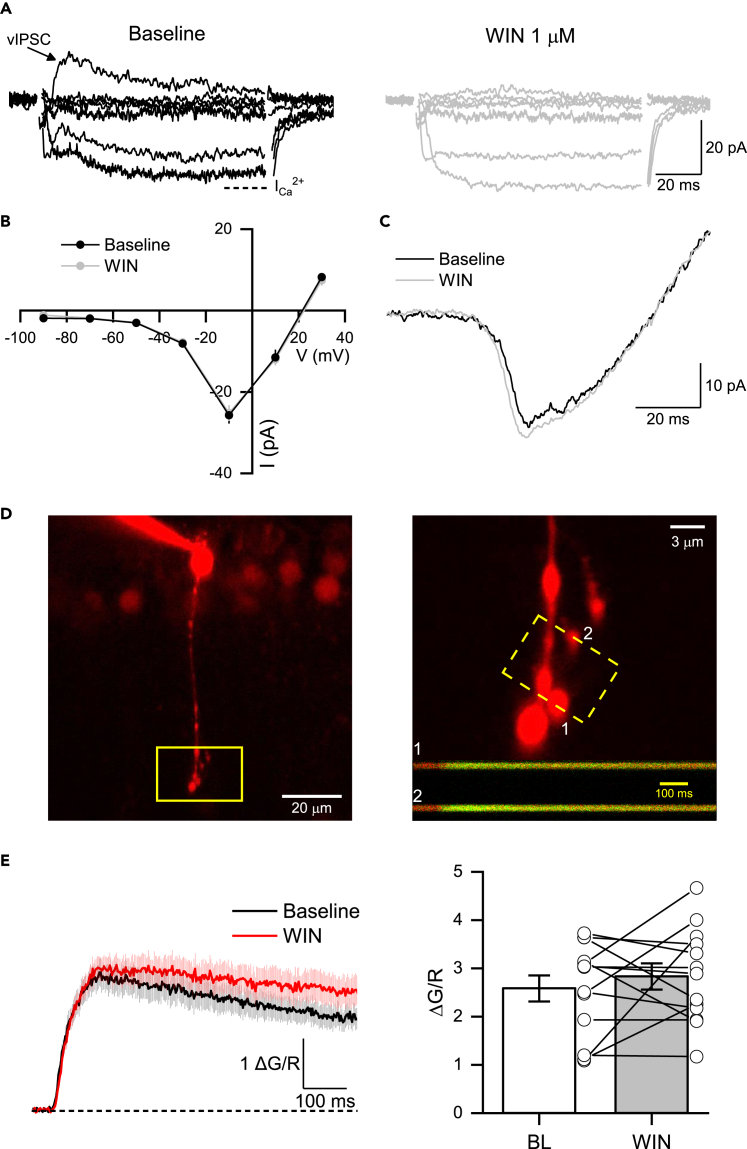
(A) Voltage-clamp recordings elicited by depolarizing steps (from −90 to +30 mV, 20 mV steps) during baseline (BL, left) and after the bath application of WIN (1 μM, right). Note that the activation of CB1R eliminated reciprocal vIPSC (black arrow) elicited at different voltage steps without affecting the amplitude of the sustained inward Ca2+ currents (ICa2+). Dotted line indicates where the ICa2+ was measured at the end of the 100 ms step pulse.
(B) Current-Voltage (I-V) curve showing that WIN had no effect on the amplitude of Ca2+ currents elicited by depolarizing voltage steps (n = 7 cells/5 animals).
(C) Representative voltage ramp depolarization from −90 to 30 mV before (black trace) and after (gray trace) the bath application of WIN.
(D) 2PLSM image of an RBC filled with Alexa Fluor 594 (left). Magnification shows RBC terminals line scanned (1 and 2) during a step-pulse depolarization (from −60 to −10 mV, 100 ms, right). Inset: Calcium transients (ΔCa2+) scanned on terminals 1 and 2 during the depolarization step.
(E) Mean ΔCa2+ evoked by step-pulse depolarization before (black) and after WIN (red) bath application (left). Summary graphs showing that WIN had no effect on ΔCa2+ amplitude (n = 13 terminals/5 cells/5 animals). Data are presented as mean ± S.E.M and open circles represent a single terminal. Comparisons were made using paired t-tests. For statistics, see Table S1.