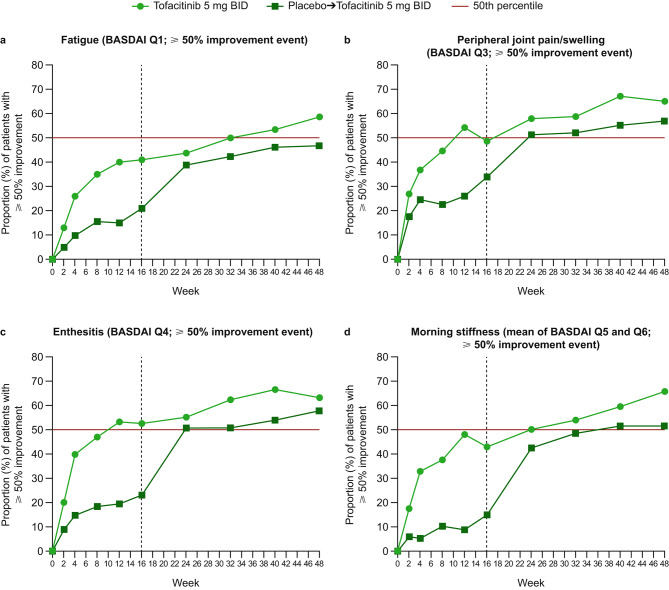
Fig. 2.
Improvement events in fatigue, peripheral outcomes, and morning stiffness. Proportions of patients with ≥ 50% improvement in a fatigue (BASDAI Q1), b peripheral joint pain/swelling (BASDAI Q3), c enthesitis (BASDAI Q4), and d morning stiffness (mean of BASDAI Q5 and Q6) at each study visit. Vertical dotted line indicates week 16, after which all patients received open-label tofacitinib until week 48. The 50th percentile line is to facilitate interpretation of the results. BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BID, twice daily; Q, question