Abstract
Endochin-like quinolones define a class of small molecule antimicrobials that target the mitochondrial electron transport chain of various human parasites by inhibiting their cytochrome bc1 complexes. The compounds have shown potent activity against a wide range of protozoan parasites including the intraerythrocytic parasites Plasmodium and Babesia, the agents of human malaria and babesiosis, respectively. First generation ELQ compounds were previously found to reduce infection by Babesia microti and Babesia duncani in animal models of human babesiosis but achieved radical cure only in combination with atovaquone and required further optimization to address pharmacological limitations. Here we report the identification of two second generation 3-Biaryl-ELQ compounds, ELQ-596 and ELQ-650, with potent antibabesial activity in vitro and favorable pharmacological properties. In particular, ELQ-598, a prodrug of ELQ-596, demonstrated high efficacy as an orally administered monotherapy at 10 mg/kg. The compound achieved radical cure in both the chronic model of B. microti-induced babesiosis in immunocompromised mice, and the lethal infection model induced by B. duncani in immunocompetent mice. Given its high potency, favorable physico-chemical properties, and low toxicity profile, ELQ-596 represents a promising drug for the treatment of human babesiosis.
Keywords: Endochin-like quinolones, Babesia, human babesiosis, B. duncani, B. microti, ELQ-596, parasite
Graphical Abstract

Human babesiosis is a severe tick-borne worldwide emerging malaria-like zoonotic disease caused by various species of Babesia. These parasites are members of the Apicomplexa phylum, which includes other important human pathogens, such as Plasmodium, the causative agent of human malaria1–4. A recent report from the US Centers for Disease Control and Prevention (CDC) highlighted a concerning trend in the distribution and prevalence of human babesiosis, with a notable rise in cases between 2011 and 2019, totaling 16,456 clinical cases across 37 states5. Eight different species of Babesia have been confirmed as causative agents of human babesiosis with clinical outcomes ranging from mild to severe4, 6,7. The majority of reported human babesiosis cases have been attributed to B. microti, particularly among immunocompromised patients1, 4, 8. However, other Babesia species, such as B. divergens and B. duncani, have been reported to cause rapid and severe infections in both immunocompetent and immunocompromised individuals9–11. Common clinical symptoms of the disease include fever, chills, sweating, myalgias, fatigue, hepatosplenomegaly, and hemolytic anemia, which can last from days to several weeks. Infections caused by B. divergens tend to be more severe and are frequently fatal if not appropriately treated7, 8. The genomes of several human Babesia parasites have recently been sequenced, assembled and annotated7, 12–19. These data revealed that these genomes exhibit distinct characteristics including smaller genome sizes, high genome compactness, and a GC content ranging between 38 and 48%, making them suitable organisms for molecular and genetic analyses13, 18. The data further revealed distinctive mechanisms of survival and adaptation, and identified significant vulnerabilities that may be targeted for the advancement of novel therapeutic strategies7, 13, 17, 18. These targets, whether they are specific proteins, metabolic pathways, or other molecular components, offer promising avenues for developing novel treatments to combat human babesiosis7. The current treatment of human babesiosis is non-specific and relies on antiparasitic medications, with two drug combinations consisting of atovaquone and azithromycin, and clindamycin and quinine3, 20. While these therapies are generally effective, alternative therapies are needed because of the emergence of resistant parasites, and undesirable side effects3, 21. Several therapeutic strategies aimed at developing more effective antibabesial therapies and to counter the emergence of drug resistant Babesia strains have been reported over the past decade3, 22, 23. For some, the main molecular target and mechanism of action have been determined, whereas for others, the mode of action remains to be elucidated.
Previous studies have demonstrated the suitability of the cytochrome b Cytb enzyme of Babesia as well as other protozoan parasites as a target for the development of new drugs24–26. The two most effective drugs targeting this enzyme are atovaquone and endochin-like quinolones (ELQs)27. Initial studies using an experimental model of chronic B. microti infection in immunocompromised mice identified the prodrug ELQ-334 as an effective antibabesial drug. However, elimination of infection in animals with no recrudescence required combining this moleculae with atovaquone28. The development of a continuous in vitro culture system for B. duncani in human erythrocytes made it possible to screen a library of first generation ELQ drugs and prodrugs in vitro prior to their evaluation in animal models of chronic and lethal B. microti and B. duncani infections, respectively29. These studies identified ELQ-502 and ELQ-331 as additional ELQs that in combination with atovaquone eliminate B. microti infection in immunocompromised mice, and protect immunocompetent C3H mice from lethal B. duncani infection30. However, monotherapy with most of these drugs resulted in recrudescence with some of the recrudescent parasites accumulating mutations in the Cytb gene conferring resistance to the drug28, 29, 31–33. Furthermore, subsequent pharmacological studies highlighted the need for further optimization of these compounds to increase solubility and reduce crystallinity.
In this study, we report on the biological activity of two second-generation ELQ derivatives, ELQ-596 and ELQ-650, and their corresponding prodrugs, ELQ-598 and ELQ-672. The compounds exhibit robust antibabesial activity in vitro alongside favorable pharmacokinetic profiles. ELQ-598, a prodrug form of ELQ-596, achieved radical cure in animal models of B. microti and B. duncani babesiosis.
RESULTS
In vitro efficacy of 2nd generation ELQs against B. duncani parasites:
A recent study by Pou and colleagues highlighted 3-position biaryl ELQ compounds as a novel class of antimalarial drugs with superior pharmacological properties over other ELQ compounds34. The compounds demonstrated excellent activity against multidrug-resistant strains of the human malaria parasite P. falciparum in vitro and enhanced efficacy in a murine model of malaria34. To evaluate the antibabesial activity of these second-generation ELQ compounds, we took advantage of the recently developed continuous in vitro culture system of B. duncani in human erythrocytes to evaluate the two lead molecules ELQ-596 and ELQ-650 and their prodrugs, ELQ-598 and ELQ-672 (Fig. 1A and 1D)34. Both ELQ-596 and its prodrug form, ELQ-598, demonstrated excellent potency in vitro, with IC50 values (50% inhibitory concentrations) of 32 ± 4.9 nM and 37 ± 2.2 nM, respectively after 62 h of incubation (Fig. 1B and 1C). Similarly, ELQ-650 and ELQ-672 exhibited IC50 values of 22 ± 4.9 nM and 57 ± 18 nM, respectively (Fig. 1E and F). We further evaluated the safety profile of these drugs and prodrugs in four human cell lines, HeLa, HepG2, HEK, and HCT116. All compounds exhibited average IC50 values greater than 10 μM, resulting in vitro therapeutic indices of more than 350, supporting their suitability as candidates for further evaluation in animal models (Table 1).
Figure 1. In vitro efficacy of active ELQs and corresponding prodrugs against B. duncani parasites.
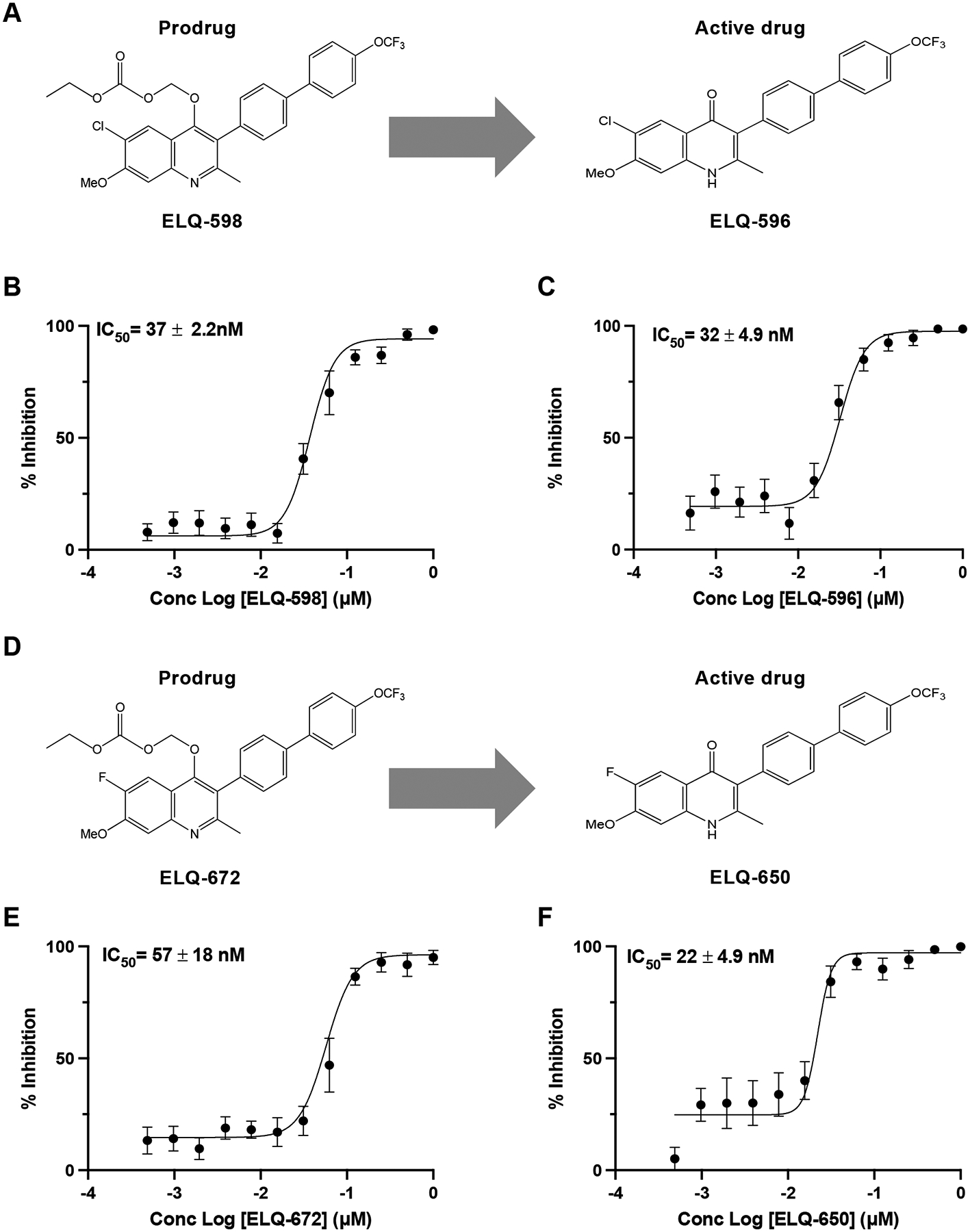
(A) Chemical structures of prodrug ELQ-598 and corresponding active drug ELQ-596 used in this study. (B) Parasite growth inhibition curve of prodrug ELQ-598. (C) Parasite growth inhibition curve of active drug ELQ-596. (D) Chemical structures of prodrug ELQ-672 and corresponding active drug ELQ-650 used in this study. (E) Parasite growth inhibition curve of prodrug ELQ-672. (F) Parasite growth inhibition curve of active drug ELQ-650. IC50 values for each compound were determined using the SYBR Green assay. The sigmoidal dose-response curves were plotted using GraphPad Prism and IC50s are represented with mean (± SD) values. Each value in the curve is the average of two different experiments with biological triplicates.
Table 1:
In vitro efficacy and therapeutic index of ELQ-596 and ELQ-650 and their prodrugs.
| Compound Name | IC50 B. duncani (nM) | IC50 Human cell lines (μM) | In vitro Therapeutic index |
|---|---|---|---|
| ELQ-596 | 32 ± 4.9 | 30 | 939 |
| ELQ-598 | 37 ± 2.2 | 19 | 529 |
| ELQ-650 | 22 ± 4.9 | 26 | 1200 |
| ELQ-672 | 57 ± 18 | 21 | 368 |
| Atovaquone | 72 ± 6 | 5 | 69 |
ELQ-598 monotherapy eliminates B. duncani in vivo and protects mice from lethal infection.
Given the promising in vitro efficacy and safety data with ELQ-596 and ELQ-650 and their respective prodrugs, we assessed the in vivo efficacy of the prodrugs in the B. duncani mouse model of lethal infection in the C3H genetic background (Fig. 2). These mice are highly susceptible to B. duncani (WA-1 isolate) infection with a mortality rate of 100% following intravenous injection of B. duncani-infected red blood cells29. As shown in Fig. 2, C3H mice infected with 103 B. duncani-infected erythrocytes and administered the vehicle alone (PEG 400) orally, succumbed to infection by day 11 post-infection (DPI 11), with parasitemia reaching approximately 6% (Fig. 2A and D). Interestingly, oral administration of ELQ-598 at 10 mg/kg daily for 5 days (DPI 3 to 7) resulted in complete elimination of the parasites in all infected animals and survival of these mice for the duration of study (45 days post-infection and 41 days post-drug removal) (Fig. 2B to D). The mice were further monitored beyond the initial endpoint of the study until DPI 84, and no parasites could be detected upon examination of Giemsa-stained thin-blood smears. Unlike ELQ-598, treatment with ELQ-672 monotherapy at 10 mg/kg from DPI 3 to DPI 7, resulted in little to no detectable parasitemia in mice by DPI 15. By DPI 16, parasites emerged and grew rapidly, reaching 8% parasitemia at DPI 19, with mice manifesting symptoms of severe disease at which time the animals were euthanized (Fig. 2C and D). Together, these data demonstrate that treatment with ELQ-598 results in the complete elimination of infection in animals, whereas ELQ-672 monotherapy delayed the infection but failed to eliminate it, with animals ultimately succumbing to infection.
Figure 2. Efficacy of ELQ-598 and ELQ-672 monotherapy against B. duncani lethal infection mouse model.

(A-D) Depicted are parasitemia graphs from female C3H/HeJ mice which were intravenously infected with 103 B. duncani-infected red blood cells. All animals were treated daily (DPI 3 – DPI 7) by oral gavage. Blood smears were made on stipulated days from each mouse and parasitemia was calculated by microscopic examination of the giemsa stained smears. Parasitemia curves with connecting lines from mice treated with the vehicle alone (PEG-400) (red) (a), ELQ-598 at 10 mg/kg (blue) (B), and ELQ-672 at 10 mg/kg (green) (C) are generated using GraphPad Prism. Survival rate of B. duncani-infected mice in the absence or following treatment with ELQ-598 or ELQ-672 derived using the Kaplan-Meier method and represented (D). ☨ indicates when an individual mouse was euthanized.
In vivo efficacy of ELQ-598 against B. microti-infected SCID mice.
We further evaluated the efficacy of ELQ-598 and ELQ-672 monotherapies in the mouse model of B. microti chronic infection in CB.17/SCID mice. Mice treated with the vehicle (PEG-400) alone reached maximum parasitemia of 60% to 70% by DPI 20 following intravenous inoculation with 104 B. microti-(LabS1) infected erythrocytes (Fig. 3A)35, whereas the group of mice treated with ELQ-598 (daily dose, 10 mg/kg from DPI 3 to 7) completely eliminated infection (Fig. 3B), with no detectable parasites by DPI 60. Conversely, ELQ-672 delayed but did not block parasite development (parasites emerged at DPI 21 compared DPI 17 in ELQ-672-treated versus vehicle-treated groups) with peak parasitemia reached at DPI 31 vs DPI 21 for ELQ-672-treated versus vehicle-treated groups (Fig. 3C). Together these data demonstrate that monotherapy with ELQ-598, but not ELQ-672, is effective at eliminating B. microti infection in mice.
Figure 3. Efficacy of ELQ-598 and ELQ-672 against B. microti-infected SCID mice.

In vivo efficacy data in B. microti-infected CB17-SCID mice following treatment with vehicle (PEG-400) alone or ELQ-598 or ELQ-672 dosed orally at a concentration of 10 mg/kg daily for 5 days. Parasitemia curves with connecting lines from the indicated times from the mice group infected with 104 B. microti-iRBCs and subsequently treated with vehicle (red) (A), or ELQ-598 10mg/kg (blue) (B), or ELQ-672 10mg/kg (green) (C) are represented.
Combination of Second-Generation ELQs with atovaquone achieves Babesia elimination.
Previous biochemical studies have demonstrated that most ELQ compounds target the ubiquinol-reduction (Qi) site of the Babesia bc1 complex whereas atovaquone targets the ubiquinol-oxidation (Qo) site30. These findings were further confirmed following the discovery of individual mutations in residues in the Qi or Qo sites responsible for resistance to either ELQ or atovaquone, respectively32. Accordingly, drug combinations consisting of ELQs and atovaquone have shown strong antibabesial efficacy in vitro and in animal models of babesiosis30. To assess the mode of interaction between second-generation ELQs and their prodrugs with atovaquone, we first calculated the FIC50 for each drug using Equation 1 as described in Methods, then we used isobologram analysis to calculate the mean fractional inhibitory concentration (ΣFIC50) using Equation 2 as described in Methods. As shown in Fig. 4, ELQ-596, ELQ-598 and ELQ-650 exhibited additive interactions with atovaquone, with ΣFIC50 between 0.5 and 1, where as ELQ-672 and atovaquone were synergistic with an ΣFIC50 of 0.5 (Table 2). Consistent with these in vitro analyses, treatment with a combination of ELQ-672 and atovaquone, at 10 mg/kg each, resulted in complete elimination of infection in SCID mice infected with B. microti as well as C3H mice infected with B. duncani, whereas monotherapies consisting of either drug were followed by parasite recrudescence (Fig. 4D and F). Not surprisingly, a combination of ELQ-598 with atovaquone also resulted in radical cure in both animal models of babesiosis (Fig. 4C and E). In the mouse model of lethal infection caused by B. duncani, all mice treated with either of the ELQs in combination with atovaquone survived by the end of the study (Fig. 4G).
Figure 4. Atovaquone + ELQ-672 combination displayed synergy in vitro and achieve cure in babesiosis mouse model.

(A-B) B. duncani parasites cultured in vitro in human red blood cells were treated with ten different concentrations of each drug by fixed ratio method. The isobologram presents the FIC50 value for each combination as well as the mean FIC (∑FIC) values of atovaquone and ELQs active and prodrugs. A) Isobolograms with FIC50 values for the combination of atovaquone+ELQ-596 (black) and atovaquone+ELQ-598 (brown) with an additive line and calculated mean ∑FIC values of 0.8 and 0.9, respectively. B) The FIC50 values for the atovaquone+ELQ-650 (black) and atovaquone+ELQ-672 (aqua blue) combinations with mean ∑FIC values of 1 and 0.5, respectively. Each data point represents the average of two independent experiments with three technical triplicates. (C-D) In vivo efficacy of the atovaquone+ELQ combinations in mice. Percent parasitemia curves obtained from the atovaquone+ELQ-598 (brown) and atovaquone+ELQ-672 (aqua blue) prodrugs combination treatment (daily oral dose of 10+10 mg/kg for 5 days) in SCID mice (female, n=5) infected with B. microti at a dose of 104 infected erythrocytes per inoculum. (E-F) In vivo efficacy of atovaquone+ELQ-598 (brown) and atovaquone+ELQ-672 (aqua blue) combination treatment (daily oral dose of 10+10 mg/kg for 5 days) in C3H/HeJ mice with 1×103 of B. duncani-infected erythrocyte infection. Parasitemia from the vehicle (PEG-400)-treated C3H or SCID mice after infection is represented with red-colored connected lines. Parasitemia (%) was calculated by microscopic analysis of Giemsa-stained blood smear samples collected at different time points following infection and treatment (a minimum of 3000 red blood cells were counted per blood smear). (G) Survival (%) of B. duncani-infected C3H/HeJ mice represented in colors consistent with the foregoing. Survival rates were calculated using the Kaplan-Meier method.
Table 2:
Isobologram analysis of the interactions between atovaquone and ELQ-596 and ELQ-650 and their prodrugs.
| Drug combinations | Mean FIC value (Σ FIC50) | Mode of Interaction |
|---|---|---|
| ATV X ELQ-598 | 0.8 | Additive |
| ATV X ELQ-596 | 0.9 | Additive |
| ATV X ELQ-672 | 0.5 | Synergistic |
| ATV X ELQ-650 | 1 | Additive |
DISCUSSION
Our in vitro and in vivo efficacy studies demonstrated that second-generation 3-Biaryl ELQs are a promising class of molecules to be added to the pipeline of therapeutic agents to be developed for the treatment of human babesiosis. In particular, the studies highlighted ELQ-598, which when used as a monotherapy, exhibited excellent biological activity in vitro and achieved complete eradication of Babesia parasites in animal models of chronic and lethal babesiosis.
Using the B. duncani continuous in vitro culture system, we showed that the active drugs, ELQ-596 and ELQ-650, and their respective prodrugs, ELQ-598 and ELQ-672, have potent activity against the parasite with calculated IC50 values in the low nM range. Studies by Pou and colleagues have shown that these compounds have excellent pharmacological properties in vitro and in mice34. Furthermore, our studies show that they have favorable safety profiles in human cell lines with TI values >300. In particular, in vivo efficacy studies with ELQ-598, showed that the drug alone administered once a day for 5 days at 10 mg/kg clears Babesia microti infection in immunocompromised mice and prevents lethal infection caused by B. duncani in immunocompetent C3H mice. While this study focused primarily on the evaluation of in vitro and in vivo efficacy at a fixed dose and course of treatment, future studies will aim to evaluate lower doses and shorter treatment durations of this compound in order to identify the optimal regimen needed to achieve radical cure, while maintain safety. Unlike, ELQ-598, ELQ-672 showed limited efficacy as a monotherapy in vivo. The compound as a monotherapy reduced time to emergence and peak parasitemia but did not eliminate infection. These findings are consistent with those reported with other classes of ELQ compounds with antibabesial activity, where structurally similar compounds were found to display major differences in biological activity28, 29, 33, and emphasize the importance of structure-activity relationship studies in selecting optimal antiparasitic candidates. Future studies are needed to investigate whether B. duncani and B. microti parasites that are refractory to ELQ-672 monotherapy are responsive to other ELQs, including ELQ-596 and ELQ-598.
While new drugs with potent activity as monotherapy are highly desirable during the early stages of drug development, the persistent emergence of drug-resistant parasites highlights the need of safeguarding new treatments through the use of synergistic or additive drug combinations. Our in vitro isobologram analyses have revealed that the interactions of 3-biaryl ELQ compounds with another bc1 complex inhibitor, atovaquone, are either additive or synergistic. Accordingly, in vivo efficacy studies have demonstrated that combination therapies consisting of ELQ-598 + atovaquone or ELQ-672 + atovaquone effectively eliminate Babesia microti and B. duncani infections in mice. Consequently, prioritizing drug combinations comprising this second generation of ELQ compounds with either atovaquone or alternative antibabesial drugs during the drug development process is imperative to mitigate the risk of treatment failure stemming from potential drug resistance emergence.
Previous studies have indicated that atovaquone is strictly a Qo site inhibitor, whereas ELQ compounds are in large part Qi site inhibitors, although as shown in the case of ELQ-502, the compound may target both sites28, 33. Available data in P. falciparum suggest that 3-biaryl-ELQs target the Qi site the parasite cytochrome bc1 complex34. Given the high conservation between the cytochrome bc1 complex of P. falciparum and those of Babesia species, it is likely that ELQ-596 and ELQ-650 have a similar mode of action in these parasites. Future research aimed at elucidating the precise mechanisms of action underlying the antiparasitic activity of these second-generation ELQ compounds against Babesia species is thus warranted. Furthermore, additional preclinical studies are needed to assess the long-term safety and tolerability of 3-biaryl-ELQs in animal models and eventually in clinical trials before such compounds can be used in babesiosis therapy in humans.
Methods:
In vitro B. duncani parasite culture and growth enumeration.
Continuous propagation of B. duncani parasite WA1 strain was conducted in DMEM/F12 medium using A+ human RBCs at 5% hematocrit. The culture medium was replaced every alternate day, and parasite growth was monitored by examining Giemsa-stained thin-blood smears by light microscopy. For determination of the inhibitory activity of experimental drugs, the SYBR Green I assay was employed as previously described. Briefly, parasite cultures (100 μl) incubated at 37°C in 96-well plates in the absence of presence of increasing concentrations of experimental drugs were mixed with an equal volume and SYBR Green-I lysis buffer (20mM Tris, pH 7.4; 5 mM EDTA, 0.008% saponin; 0.08% Triton X-100; and 1x SYBR Green I (Sigma, 7567)). Fluorescent readings were taken on a BioTek SynergyMX plate reader with excitation at 497 nm and emission at 520 nm.
Animal Studies.
Immunocompetent C3H/HeJ and immunocompromised (CB17/SCID) mice were purchased from The Jackson Laboratory and Charles River Laboratories, respectively. All animal experiments adhered to the Yale University institutional guidelines for the care and use of laboratory animals. The protocols were approved by the Institutional Animal Care and Use Committees (IACUC) at Yale University.
In vitro drug efficacy assays.
The effectiveness of ELQs on B. duncani in vitro parasite growth was assessed by investigating the intra-erythrocytic development cycle (IDC) inhibition and determining the IC50 through an established protocol36. Briefly, the in vitro parasite culture (0.1% parasitemia), continuously treated with 2-fold serially diluted concentrations of ELQs in a 96-well plate over three generations (62 hours). Following treatment, parasitemia was quantified using the SYBR Green-I method (above mentioned). Background fluorescence, obtained from uninfected RBCs in a complete medium, was subtracted from each well containing parasites. The IC50 value determined from the sigmoidal dose-response curve by plotting the drug log concentration against percent parasite growth using GraphPad Prism v9.4.1. Each IC50 value, obtained from two independent experiments with biological triplicates, is presented as mean ± SD.
Drug cytotoxicity evaluation.
The cytotoxicity of ELQ compounds was tested on human cell lines (HeLa, HepG2, HEK, and HCT116) obtained from the American Type Culture Collection (ATCC) and maintained in complete Dulbecco’s Modified Eagle’s Medium (DMEM) (Invitrogen 11995–065), as described previously30. In a 96-well plate, 20,000 cells per well were seeded and treated with 2-fold serially diluted respective ELQs, incubated at 37°C for 48 hours. After the drug treatment, cells were incubated with MTT reagent (M6494) for 4 hours, and absorbance was measured at OD590 nm using the BioTek Synergy™ Mx Microplate Reader. From the obtained absorbance values, percent cell viability was calculated, and IC50 values were determined from nonlinear regression dose-response curves by plotting the data using GraphPad Prism v9.4.1.
In vivo drug efficacy in mice infected with B. duncani and B. microti.
Animal drug efficacy studies were conducted using standardized assays as reported previously35. Briefly, female C3H/HeJ mice (6 weeks old, 5 mice in each group) were intravenously infected with 1×103 B. duncani (WA1)-infected RBCs. Treatment was administered daily for 5 days (DPI 3 to 7) by oral gavage, starting on the 3rd day post-infection (DPI 3). Animals received 100 μl of the vehicle alone (PEG 400), atovaquone (10 mg/kg), ELQ-598 (10 mg/kg), ELQ-672 (10 mg/kg), or a combination of individual ELQs plus atovaquone (10 mg/kg each). Female CB.17/SCID mice (6 weeks old, 5 mice in each group) were intravenously infected with 1×104 B. microti (LabS1)-infected RBCs. Treatment was administered daily for 5 days by oral gavage, starting on the 3rd day post-infection. Animals received 100 μl of the vehicle alone (PEG 400), atovaquone (10 mg/kg), ELQ-598 (10 mg/kg), ELQ-672 (10 mg/kg), or a combination of individual ELQs plus atovaquone (10 mg/kg each).
Evaluation of in vitro drug-drug interactions by isobologram analysis.
To understand the type of interaction between ELQs and antiparasitic drugs, we conducted fixed-ratio drug combination experiments following a previously described protocol30. Briefly, combinations of the drugs of interest were tested using ten fixed ratios (10:0, 9:1, 8:2, 7:3, 6:4, 5:5, 4:6, 3:7, 2:8, 1:9, and 0:10). The starting concentration was fixed at 8 times the IC50 value for each single dose of the drug, and then serially diluted 2-fold. In a 96-well plate, parasites (0.1% parasitemia in 5% hematocrit) were incubated at 37°C for 60 hours with each drug combination. The half-maximal inhibitory concentration (IC50) was determined from a sigmoidal dose-response curve. The fractional inhibitory concentration (FIC50) of each drug at different ratios was calculated using equation 1.
The interaction between drugs derived from the sum of FIC50 (ΣFIC50) using equation 2.
Finally, to determine the mode of interactions based on pharmacologically accepted drug-drug interaction studies (ΣFIC50 < 0.8 = synergy, 0.8 ≤ ΣFIC50 < 1.4 = additive, and ΣFIC50 ≥ 1.4 = antagonistic interactions), isobolograms were plotted using the FIC50 values from each combination. Data were averaged from at least two independent experiments, each run-in triplicates. Analysis was carried out using GraphPad Prism v9.4.1.
Acknowledgments
Research in the CBM Lab is supported by the National Institutes of Health grants (AI123321, AI138139, AI152220, and AI136118), the Steven and Alexandra Cohen Foundation (Lyme 62 2020), the Global Lyme Alliance and the NBIA Foundations. This project was also supported with funds from the United States Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development Program Award number i01 BX003312 (M.K.R.). M.K.R. is a recipient of a VA Research Career Scientist Award (14S-RCS001). Research reported in this publication was also supported by the US National Institutes of Health under award numbers R01AI100569 and R01AI141412 (M.K.R.) and by the U.S. Department of Defense Peer Reviewed Medical Research Program (PR181134) (M.K.R.). This work was also funded by VA Merit Review Award BX004522 to JSD from the US Department of Veterans Affairs Biomedical Laboratory Research and Development. The National Science Foundation provided instrument funding for the BioAnalytical Mass Spectrometry Facility at Portland State University (NSF, MRI 1828573) which was used to generate HRMS analytical measurements. The authors would like to acknowledge the contributions of the OHSU Medicinal Chemistry Core (Research Resource ID: SCR 019048).
Footnotes
Conflict of Interest Statement
Choukri Ben Mamoun, Pratap Vydyam and Meenal Chand declare no conflict of interest. Sovitj Pou, Rolf W. Winter, Katherine M. Liebman, Aaron Nilsen, J. Stone Doggett, Michael K. Riscoe are listed as co-inventors of technology that is involved in this research and which has been licensed by OHSU for commercial development. This potential conflict of interest has been reviewed and managed by OHSU and the Portland VAMC.
REFERENCES:
- (1).Krause PJ Human babesiosis. Int J Parasitol 2019, 49 (2), 165–174. DOI: 10.1016/j.ijpara.2018.11.007 From NLM. [DOI] [PubMed] [Google Scholar]
- (2).Florin-Christensen M; Schnittger L Piroplasmids and ticks: a long-lasting intimate relationship. Front Biosci (Landmark Ed) 2009, 14 (8), 3064–3073. DOI: 10.2741/3435 From NLM. [DOI] [PubMed] [Google Scholar]
- (3).Renard I; Ben Mamoun C Treatment of Human Babesiosis: Then and Now. Pathogens 2021, 10 (9). DOI: 10.3390/pathogens10091120 From NLM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Vannier E; Krause PJ Human babesiosis. N Engl J Med 2012, 366 (25), 2397–2407. DOI: 10.1056/NEJMra1202018 From NLM. [DOI] [PubMed] [Google Scholar]
- (5).Swanson M, P. A, Williamson J, Montgomery S. Trends in Reported Babesiosis Cases United States, 2011–2019. CDC MMWR Morb Mortal 2023, 2023;72:273–277 (Wkly Rep). DOI: 10.15585/mmwr.mm7211a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Wang Y; Zhang S; Li X; Nian Y; Liu X; Liu J; Yin H; Guan G; Wang J A highresolution melting approach for the simultaneous differentiation of five human babesiosis-causing Babesia species. Parasit Vectors 2023, 16 (1), 299. DOI: 10.1186/s13071-023-05839-5 From NLM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Singh P; Vydyam P; Fang T; Estrada K; Gonzalez LM; Grande R; Kumar M; Chakravarty S; Berry V; Ranwez V; Carcy B; Depoix D; Sánchez S; Cornillot E; Abel S; Ciampossin L; Lenz T; Harb O; Sanchez-Flores A; Montero E; Le Roch KG; Lonardi S; Ben Mamoun C Multiomics analysis reveals B. MO1 as a distinct Babesia species and provides insights into its evolution and virulence. bioRxiv 2024. DOI: 10.1101/2024.01.17.575932 From NLM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Krause PJ; Auwaerter PG; Bannuru RR; Branda JA; Falck-Ytter YT; Lantos PM; Lavergne V; Meissner HC; Osani MC; Rips JG; Sood SK; Vannier E; Vaysbrot EE; Wormser GP Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA): 2020 Guideline on Diagnosis and Management of Babesiosis. Clin Infect Dis 2021, 72 (2), 185–189. DOI: 10.1093/cid/ciab050 From NLM. [DOI] [PubMed] [Google Scholar]
- (9).Kjemtrup AM; Conrad PA Human babesiosis: an emerging tick-borne disease. Int J Parasitol 2000, 30 (12–13), 1323–1337. DOI: 10.1016/s0020-7519(00)00137-5 From NLM. [DOI] [PubMed] [Google Scholar]
- (10).Kukina IV; Guzeeva TM; Zelya OP; Ganushkina LA Fatal human babesiosis caused by Babesia divergens in an asplenic host. IDCases 2018, 13, e00414. DOI: 10.1016/j.idcr.2018.e00414 From NLM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Herc E; Pritt B; Huizenga T; Douce R; Hysell M; Newton D; Sidge J; Losman E; Sherbeck J; Kaul DR Probable Locally Acquired Babesia divergens-Like Infection in Woman, Michigan, USA. Emerg Infect Dis 2018, 24 (8), 1558–1560. DOI: 10.3201/eid2408.180309 From NLM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Cornillot E; Dassouli A; Garg A; Pachikara N; Randazzo S; Depoix D; Carcy B; Delbecq S; Frutos R; Silva JC; Sutton R; Krause PJ; Mamoun CB Whole genome mapping and re-organization of the nuclear and mitochondrial genomes of Babesia microti isolates. PLoS One 2013, 8 (9), e72657. DOI: 10.1371/journal.pone.0072657 From NLM Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Cornillot E; Hadj-Kaddour K; Dassouli A; Noel B; Ranwez V; Vacherie B; Augagneur Y; Brès V; Duclos A; Randazzo S; Carcy B; Debierre-Grockiego F; Delbecq S; Moubri-Ménage K; Shams-Eldin H; Usmani-Brown S; Bringaud F; Wincker P; Vivarès CP; Schwarz RT; Schetters TP; Krause PJ; Gorenflot A; Berry V; Barbe V; Ben Mamoun C Sequencing of the smallest Apicomplexan genome from the human pathogen Babesia microti†. Nucleic Acids Research 2012, 40 (18), 9102–9114. DOI: 10.1093/nar/gks700 (acccessed 9/26/2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Cuesta I; Gonzalez LM; Estrada K; Grande R; Zaballos A; Lobo CA; Barrera J; Sanchez-Flores A; Montero E High-Quality Draft Genome Sequence of Babesia divergens, the Etiological Agent of Cattle and Human Babesiosis. Genome Announc 2014, 2 (6). DOI: 10.1128/genomeA.01194-14 From NLM PubMed-not-MEDLINE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Garg A; Stein A; Zhao W; Dwivedi A; Frutos R; Cornillot E; Ben Mamoun C Sequence and annotation of the apicoplast genome of the human pathogen Babesia microti. PLoS One 2014, 9 (10), e107939. DOI: 10.1371/journal.pone.0107939 From NLM Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Gonzalez LM; Estrada K; Grande R; Jimenez-Jacinto V; Vega-Alvarado L; Sevilla E; Barrera J; Cuesta I; Zaballos A; Bautista JM; Lobo CA; Sanchez-Flores A; Montero E Comparative and functional genomics of the protozoan parasite Babesia divergens highlighting the invasion and egress processes. PLoS Negl Trop Dis 2019, 13 (8), e0007680. DOI: 10.1371/journal.pntd.0007680 From NLM Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Jackson AP; Otto TD; Darby A; Ramaprasad A; Xia D; Echaide IE; Farber M; Gahlot S; Gamble J; Gupta D; Gupta Y; Jackson L; Malandrin L; Malas TB; Moussa E; Nair M; Reid AJ; Sanders M; Sharma J; Tracey A; Quail MA; Weir W; Wastling JM; Hall N; Willadsen P; Lingelbach K; Shiels B; Tait A; Berriman M; Allred DR; Pain A The evolutionary dynamics of variant antigen genes in Babesia reveal a history of genomic innovation underlying host-parasite interaction. Nucleic Acids Res 2014, 42 (11), 7113–7131. DOI: 10.1093/nar/gku322 From NLM Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Singh P; Lonardi S; Liang Q; Vydyam P; Khabirova E; Fang T; Gihaz S; Thekkiniath J; Munshi M; Abel S; Ciampossin L; Batugedara G; Gupta M; Lu XM; Lenz T; Chakravarty S; Cornillot E; Hu Y; Ma W; Gonzalez LM; Sánchez S; Estrada K; Sánchez-Flores A; Montero E; Harb OS; Le Roch KG; Mamoun CB Babesia duncani multi-omics identifies virulence factors and drug targets. Nat Microbiol 2023. DOI: 10.1038/s41564-023-01360-8 From NLM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Virji AZ; Thekkiniath J; Ma W; Lawres L; Knight J; Swei A; Roch KL; Mamoun CB Insights into the evolution and drug susceptibility of Babesia duncani from the sequence of its mitochondrial and apicoplast genomes. Int J Parasitol 2019, 49 (2), 105–113. DOI: 10.1016/j.ijpara.2018.05.008 From NLM Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Smith RP; Hunfeld KP; Krause PJ Management strategies for human babesiosis. Expert Rev Anti Infect Ther 2020, 18 (7), 625–636. DOI: 10.1080/14787210.2020.1752193 From NLM. [DOI] [PubMed] [Google Scholar]
- (21).Marcos LA; Leung A; Kirkman L; Wormser GP Use of tafenoquine to treat a patient with relapsing babesiosis with clinical and molecular evidence of resistance to azithromycin and atovaquone. IDCases 2022, 27, e01460. DOI: 10.1016/j.idcr.2022.e01460 From NLM PubMed-not-MEDLINE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Hildebrandt A; Zintl A; Montero E; Hunfeld KP; Gray J Human Babesiosis in Europe. Pathogens 2021, 10 (9). DOI: 10.3390/pathogens10091165 From NLM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Hildebrandt A; Gray JS; Hunfeld KP Human babesiosis in Europe: what clinicians need to know. Infection 2013, 41 (6), 1057–1072. DOI: 10.1007/s15010-013-0526-8 From NLM. [DOI] [PubMed] [Google Scholar]
- (24).Winter RW; Kelly JX; Smilkstein MJ; Dodean R; Hinrichs D; Riscoe MK Antimalarial quinolones: synthesis, potency, and mechanistic studies. Exp Parasitol 2008, 118 (4), 487–497. DOI: 10.1016/j.exppara.2007.10.016 From NLM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Doggett JS; Nilsen A; Forquer I; Wegmann KW; Jones-Brando L; Yolken RH; Bordón C; Charman SA; Katneni K; Schultz T; Burrows JN; Hinrichs DJ; Meunier B; Carruthers VB; Riscoe MK Endochin-like quinolones are highly efficacious against acute and latent experimental toxoplasmosis. Proc Natl Acad Sci U S A 2012, 109 (39), 15936–15941. DOI: 10.1073/pnas.1208069109 From NLM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Winter R; Kelly JX; Smilkstein MJ; Hinrichs D; Koop DR; Riscoe MK Optimization of endochin-like quinolones for antimalarial activity. Exp Parasitol 2011, 127 (2), 545–551. DOI: 10.1016/j.exppara.2010.10.016 From NLM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Nilsen A; Miley GP; Forquer IP; Mather MW; Katneni K; Li Y; Pou S; Pershing AM; Stickles AM; Ryan E; Kelly JX; Doggett JS; White KL; Hinrichs DJ; Winter RW; Charman SA; Zakharov LN; Bathurst I; Burrows JN; Vaidya AB; Riscoe MK Discovery, synthesis, and optimization of antimalarial 4(1H)-quinolone-3-diarylethers. J Med Chem 2014, 57 (9), 3818–3834. DOI: 10.1021/jm500147k From NLM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Lawres LA; Garg A; Kumar V; Bruzual I; Forquer IP; Renard I; Virji AZ; Boulard P; Rodriguez EX; Allen AJ; Pou S; Wegmann KW; Winter RW; Nilsen A; Mao J; Preston DA; Belperron AA; Bockenstedt LK; Hinrichs DJ; Riscoe MK; Doggett JS; Ben Mamoun C Radical cure of experimental babesiosis in immunodeficient mice using a combination of an endochin-like quinolone and atovaquone. J Exp Med 2016, 213 (7), 1307–1318. DOI: 10.1084/jem.20151519 From NLM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Pal AC; Renard I; Singh P; Vydyam P; Chiu JE; Pou S; Winter RW; Dodean R; Frueh L; Nilsen AC; Riscoe MK; Doggett JS; Ben Mamoun C Babesia duncani as a Model Organism to Study the Development, Virulence, and Drug Susceptibility of Intraerythrocytic Parasites In Vitro and In Vivo. J Infect Dis 2022, 226 (7), 1267–1275. DOI: 10.1093/infdis/jiac181 From NLM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Chiu JE; Renard I; Pal AC; Singh P; Vydyam P; Thekkiniath J; Kumar M; Gihaz S; Pou S; Winter RW; Dodean R; Frueh L; Nilsen AC; Riscoe MK; Doggett JS; Ben Mamoun C Effective Therapy Targeting Cytochrome bc(1) Prevents Babesia Erythrocytic Development and Protects from Lethal Infection. Antimicrob Agents Chemother 2021, Aac0066221. DOI: 10.1128/aac.00662-21 From NLM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Alday PH; Bruzual I; Nilsen A; Pou S; Winter R; Ben Mamoun C; Riscoe MK; Doggett JS Genetic Evidence for Cytochrome b Qi Site Inhibition by 4(1H)-Quinolone-3-Diarylethers and Antimycin in Toxoplasma gondii. Antimicrob Agents Chemother 2017, 61 (2). DOI: 10.1128/AAC.01866-16 From NLM Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Chiu JE; Renard I; George S; Pal AC; Alday PH; Narasimhan S; Riscoe MK; Doggett JS; Ben Mamoun C Cytochrome b Drug Resistance Mutation Decreases Babesia Fitness in the Tick Stages But Not the Mammalian Erythrocytic Cycle. J Infect Dis 2022, 225 (1), 135–145. DOI: 10.1093/infdis/jiab321 From NLM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Chiu JE; Renard I; Pal AC; Singh P; Vydyam P; Thekkiniath J; Kumar M; Gihaz S; Pou S; Winter RW; Dodean R; Frueh L; Nilsen AC; Riscoe MK; Doggett JS; Mamoun CB Effective Therapy Targeting Cytochrome bc(1) Prevents Babesia Erythrocytic Development and Protects from Lethal Infection. Antimicrob Agents Chemother 2021, 65 (9), e0066221. DOI: 10.1128/AAC.00662-21 From NLM Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Sovitj P; Rolf WW; Rozalia AD; Katherine L; Yuexin L; Mather MW; Binod N; Aaron N; Mason JH; Teresa MR; Sydney L; Jane XK; Martin JS; Sandhya K; Akhil BV; P HA; J SD; Michael KR 3-Position Biaryl Endochin-Like Quinolones with Enhanced Antimalarial Performance. 2024. [DOI] [PMC free article] [PubMed]
- (35).Vydyam P; Pal AC; Renard I; Chand M; Kumari V; Gennaro JC; Mamoun CB Tafenoquine-Atovaquone Combination Achieves Radical Cure and Confers Sterile Immunity in Experimental Models of Human Babesiosis. J Infect Dis 2024, 229 (1), 161–172. DOI: 10.1093/infdis/jiad315 From NLM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Abraham A; Brasov I; Thekkiniath J; Kilian N; Lawres L; Gao R; DeBus K; He L; Yu X; Zhu G; Graham MM; Liu X; Molestina R; Ben Mamoun C Establishment of a continuous in vitro culture of Babesia duncani in human erythrocytes reveals unusually high tolerance to recommended therapies. J Biol Chem 2018, 293 (52), 19974–19981. DOI: 10.1074/jbc.AC118.005771 From NLM. [DOI] [PMC free article] [PubMed] [Google Scholar]


