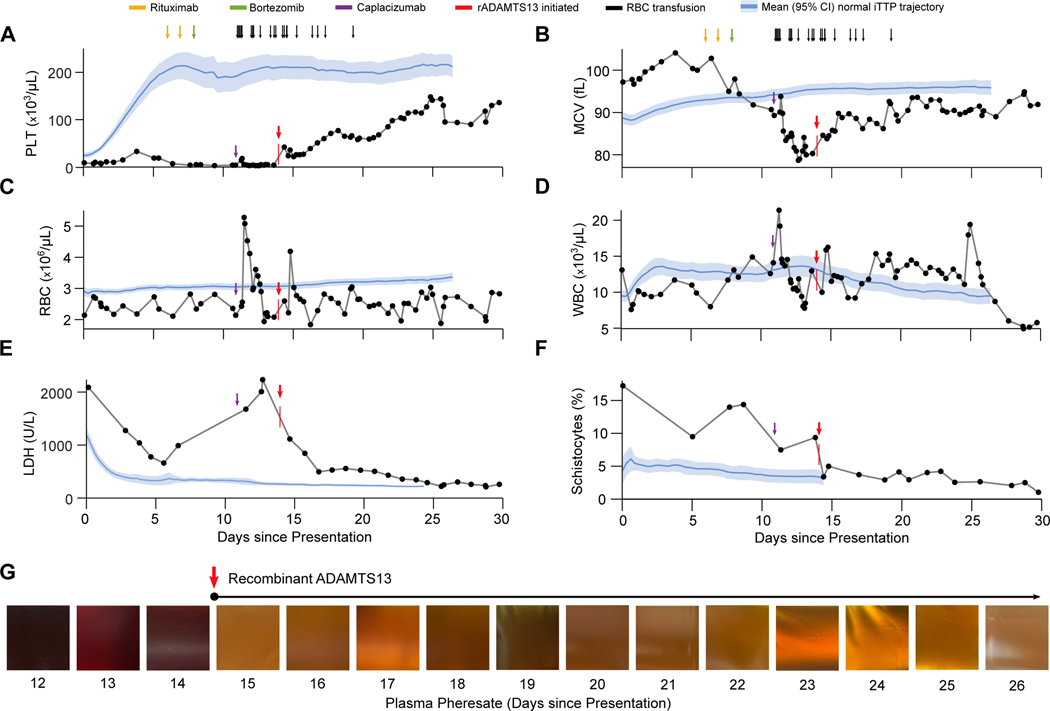
Figure 2. Laboratory Parameters Before and After Administration of Recombinant ADAMTS13.
Panels A through F depict the trajectories of key laboratory values for this patient in relation to the noted clinical events. The vertical red arrows and marks denote initiation of rADAMTS13 treatment at 80 IU/kg intravenously every 12 hours. Blue lines and shaded areas represent the corresponding mean ± 95% CI values for 102 consecutive iTTP patients in the Harvard TMA Research Collaborative dataset. The patient received daily plasma exchange treatments throughout the period shown. Panel G shows the gross appearance of the patient’s plasma pheresate at the conclusion of daily plasma exchange treatments on days 12–26. The purple arrow marks the administration of caplacizumab, while the red arrow denotes the initiation of rADAMTS13. Abbreviations: PLT, platelet count; RBC, red blood cell count; LDH, lactate dehydrogenase; MCV, mean corpuscular volume; WBC, white blood cell count.